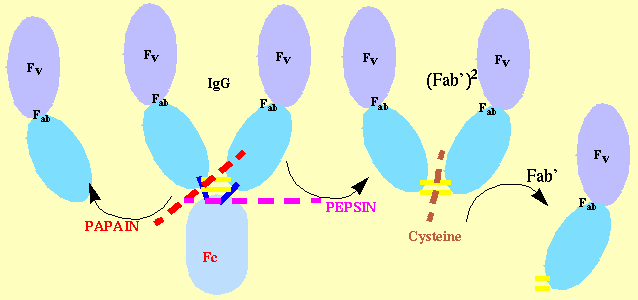
Fab Cleavage:
33. Stura E.A., Fieser G.G., Wilson I.A. (1993) Crystallization of Antibodies and Antibody-Antigen Complexes. Immunomethods 3, 164-179.
Abstract:
Although many antibodies have been crystallized, the number of structures determined in
both their complexed and unliganded forms remains relatively small. With the recent improvements in
he use of molecular replacement (MR),
the structure determination of Fabs and Fab complex structures can proceed more rapidly,
but crystallization often remains a major obstacle. Substantial improvements in methodologies have
helped with the success rate
in the crystallizations of Fabs and Fab-antigen complexes that are beyond previous expectations.
Crystallization and structure determination has been directed mainly towards Fab fragments.
The reason for this choice
remains linked both to the ease with which the structure of Fabs can be determined and to
the difficulties that have been presented by the crystallization of whole immunoglobulins.
Such difficulty is currently believed to be due to flexibility or conformat
tional heterogeneity of the IgG as well as the added heterogeneity from the glycosylation of the Fc fragment.
Fabs share some of the same problems mainly because of the degree of heterogeneity that is the result of the
proteolytic cleavage used to fragment
Fabs share some of the same problems mainly because of the degree of heterogeneity that is the result
of the proteolytic cleavage used to fragment the immunoglobulins, the flexibility in elbow regions and
in some cases from glycosylation. A systematic app
roach to the cleavage, purification, and analysis of the resultant product can yield immunoglobulin
fragments amenable to crystallization. A rational
screening of crystallization conditions with extensive use of seeding can in most cases enable
progress from small microcrystalline aggregates to
large X-ray quality crystals. Such methodologies have become so effective that Fabs are now
being used as tools to aid in the crystallization of
other molecules which have been found difficult to crystallize by themselves.
Preparation and Cleavage of Immunoglobulins
Separation of IgG and cleavage of Fabs - IgG are purified from ascitic fluid by a 50% saturated
ammonium sulfate precipitation. The precipitated material, often containing greater than 95% in
IgG, is then dialyzed against 100mM sodium acetate or phosphate-buffered saline (PBS)
(see also Perkins Elmer site). A
search to determine optimal cleavage conditions is carried out using papain or pepsin. In practice
both proteases are tried experimentally at several concentrations and for several different digestion times and the best combination selected on the basis the analysis of the products by sodium
dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). The proteolytic cleavage
is initially optimized to maximize the yield of Fab or F(ab')2.
Papain Cleavage -
Mercuripapain is pre-activated with 10mM cysteine in 1.25mM EDTA for 15 minutes at 37C, and then added to the IgG
(5-10 mg/ml) at a 1:50 to 1:200 (w/w) ratio enzyme to antibody. A series of incubations are carried
out at 37C for 15min-12 hours to determine the susceptibility of the antibody to cleavage. The
cleavage is stopped by the addition of iodoacetamide (20-50 mM). Optimal conditions are determined by analysis of the products of the enzymatic cleavage by SDS-PAGE. In the
case of an Epstein Barr Virus neutralizing antibody, 72A1 cleavage with papain at pH 5.5, but not at pH 7.0, produced fragments which did not bind
to the surface glycoprotein. Although this may be specific for this antibody, care should be taken
when cleaving with proteases and binding activities checked before and after cleavage.
Pepsin Cleavage -
Pepsin at a typical concentration of 50mg/ml is used to digest the antibody.The
period for which the reaction is allowed to proceed again varies, from 15 minutes to overnight for
individual antibodies. Pepsin cleavage is carried out at pH 5.5, but it may be lowered to increase
cleavage for more resilient antibodies. The reaction is stopped by raising the pH to neutrality. Pepsin yields typically F(ab')2 (Figure 1b; lane 2) fragments which are then reduced and alkylated to
yield Fab' fragments with ~10mM cysteine at 25C. Again, different antibodies show differential susceptibility to reduction and to the cysteine concentration and incubation
time needed to optimize the yield of Fab. The reduced sulfhydryls are acetylated with iodoacetamide for a period of a few hours in the absence of light. Optimization of this reduction step may
be critical for successful crystallization. In our experience, as seen with the anti-progesterone
antibody DB3, it was better to under-reduce rather than over-reduce the preparation. In
this particular case, it was found that a small amount of unreduced F(ab')2, had little effect on the
crystallization, while over-reduction resulted in crystals of smaller size. Indeed, reduction of some
of the intra-chain disulfides that are important for the integrity is likely to produce a heterogeneous product, which unlike the F(ab')2, can easily be incorporated in the reticulum of the growing crystal and produce lattice defects.
Elastase Cleavage -
Although elastase is not usually the protease of choice in the cleavage of
immunoglobulins, this enzyme has proved to be useful in cases where cleavage with the other proteases has resulted in excessive fragmentation of the immunoglobulin. The cleavage product with
elastase can be either an Fab or even an F(ab')2 which is reduced, as for pepsin cleavage, to
produce an Fab'. Other proteases may prove to be useful in non-standard cases.
Purification of Fab Fragments - Both cleavage and reduction steps have to be optimized to yield
as homogeneous a product as possible. Unfortunately, for most Fab and Fab' preparations,
uncleaved IgG, unreduced F(ab')2, and over-reduced Fab even in the best of situations. Purification is needed for most Fab samples. The digested material is applied to a size exclusion column
with 0.1M sodium acetate pH 5.5. Although the choice of buffer is arbitrary, and PBS has been
satisfactory in many cases, 0.1M sodium acetate pH 5.5 has been used routinely in our laboratory
to avoid precipitation of the Fab on the size exclusion column, which may occur with neutral or
alkaline buffers. The cleaved fragments of immunoglobulins, Fab and Fab', often have isoelectric
points and reduced solubility above pH 7. The fractions recovered are analyzed both by absor
bance measurement at 280 nm and checked for purity by SDS-PAGE and by isoelectric focusing
gel electrophoresis (IEF). Fractions containing the purified Fab are usually pooled and
concentrated. Depending on the heterogeneity of the sample, a preliminary crystallization screen
ing may be performed at this stage. In most cases, ion exchange chromatography purification of
the Fab will be required and may be performed using a Mono S Mono Q or Mono P columns. The initial attempts at this
purification will follow the manufacturer's suggestions based on the isoelectric point of the Fab or
F(ab')2, but frequently the ideal buffer conditions for resolving the multiple species must be deter
mined empirically. The elution gradient used usually consists of 0 to 1M ammonium sulfate or
sodium chloride using the same buffer in which the column has been pre-equilibrated or a pH gradient. The various fractions obtained are analyzed by IEF to determine purity. Reactivity is checked with the antigen and catalytic activity monitored in the case of abzymes. These
fractions may be pooled or each fraction concentrated separately. The concentrated pooled frac
tions are tested for crystallizability individually with and without antigen. This overall approach
to purification and crystallization screening is applied to all Fab, F(ab')2 and Fab' fragments and
occasionally to intact immunoglobulins.
IgM separation and cleavage -
The crystallization of intact an IgM has not been reported to date.
Although we have obtained microcrystals of fragments from IgM and are currently involved in
studies to better understand their cleavage and subsequent crystallization, we refer to the use of
papain for the production of crystallizable fragments from human IgM as described by Newkirk et
al. [for rabbit IgM see McIlroy et al.] which may be preferable to the procedures using
trypsin and pepsin. Beale and Van Dort should be consulted for a general review of
the use of the various proteases and the many different cleavage fragments that are obtained from
the cleavage of IgM. Preparation of intact IgM consists of a two step method. The IgM is first pre
cipitated by dialyzing against cold distilled water with at least two changes over 24 hours. While
this procedure works for many IgMs, not all will precipitate out at low ionic strength. Precipita
tion with 50% saturated ammonium sulfate should be attempted for others. The precipitate is cen
trifuged and then resuspended in buffer, typically PBS. The preparation may contain impurities
such as transferrin and albumin. For further purification it is run over a size exclusion column. Since IgMs (M.W.~900kDa) are much larger than
most other contaminating proteins, this procedure results in a relatively uncontaminated prepara
tion. The procedure for the cleavage of IgM does not differ substantially for the protocol
described above for papain cleavage.

