Like solid-state NMR, infrared (vibrational) spectroscopy can be done on intact cell walls provided that they can be prepared as thin enough layers to give suitable absorbance values. The mid-range infrared spectra carry a large amount of structural information, although they are not as readily interpretable as 13C NMR spectra are and different polysaccharides can give disconcertingly similar FTIR spectra.
A particular advantage of FTIR microscopy is that when carried out with polarised radiation, it can provide information on the orientation of polymers or sometimes of specific bonds within them. This methodology has been worked out by Reg Wilson and Maureen McCann at Norwich. We are currently attempting to put it on a rigorous quantitative basis for the hydrogen-bonded hydroxyl groups in cellulose, using as a baseline the neutron diffraction structure of cellulose II from Chanzy's lab which has reasonably accurate proton positions. More simply, the C-O-C stretching vibrational mode across the glycosidic linkage of a polymer is a reliable indication of the chain orientation, which obviously relates to the way in which the polymer can carry internal stresses within the architecture of the cell wall.
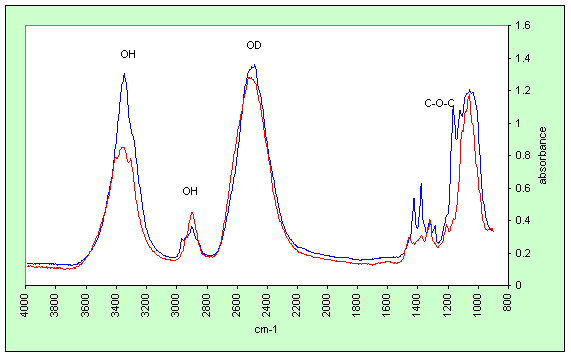
By deuteration of free hydroxyl groups with D2O in either liquid or vapour phase we can distinguish internal chains of cellulose microfibrils, which do not deuterate at neutral pH, from surface chains of cellulose and other polysaccharides that deuterate readily.
Accurate polarisation data require good-quality, undistorted spectra. The key to obtaining spectra of the necessary quality is the samples themselves. Isabelle His, who in a previous project acquired a great deal of skill in sectioning difficult plant materials for TEM, has been adapting the TEM methodology to prepare very uniform sections about 1 mm thick for FTIR microscopy.

Polarised FTIR spectra of a single flax
fibre cell deuterated with D2O.
Blue: polarisation parallel to fibre axis.
Red: polarisation transverse to fibre axis.