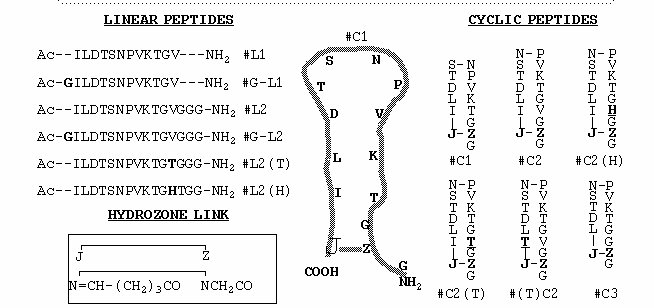
Abstract: X-ray quality crystals of a Fab fragment from a transmission-blocking monoclonal antibody 4B7 (MAb 4B7) that was formed against a sexual stage protein Pfs25 of Plasmodium falciparum were grown as uncomplexed and peptide complexed forms. Because cleavage with pepsin or papain produced a non-homogeneous product, the whole immunoglobulin was crystallized (Stura et al. companion article1). Subsequently, a Fab fragment was obtained using the protease, elastase. The best complex crystals were obtained using cyclic peptide from the third EGF-like domain of Pfs25, where the N and C termini are joined with a covalent hydrogen-bond mimic. MAb 4B7 binds a linear peptide, however, the cyclic peptide shows a significant increase in binding affinity. An initial data set, complete to 3.0Å, has been collected from these complex crystals. The packing arrangement of the Fab in the crystals has been determined by molecular replacement and refinement of the structure is in progress. The genes for the variable domain of the Fab were cloned, sequenced and the primary amino acid sequence deduced. Knowledge of the three-dimensional structure of this Fab-peptide complex will be important in the understanding the mode by which this antibody recognizes and prevents transmission of the parasite.
34. Stura E.A., Kang, A.S, Stefanko, R.S., Calvo, J, Gaarder, K.L, Kaslow D.C., and Satterthwait, A.C. (1993) Crystallographic studies of transmission blocking antimalaria Fab 4B7 with cyclic and linear peptides from the Pfs25 protein of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene 49:Suppl.(137)-177.
Abstract: X-ray quality crystals of a transmission-blocking monoclonal antibody 4B7 (MAb 4B7) against a sexual stage protein Pfs25 of Plasmodium falciparum have been obtained. Both the intact immunoglobulin and an elastase produced Fab fragment have been crystallized both free and complexed with cyclic and linear peptides. While MAb 4B7 binds a linear peptide, cyclic peptides modeled on a predicted b-hairpin loop of the third EGF-like domain of Pfs25 bind better and are readily co-crystallized with the Fab. Several cyclic peptides and their linear counterparts have been synthesized. The affinity of MAb 4B7 for the different peptides varies widely. X-ray data have been collected from various peptide-complexed and free Fab crystals. The packing arrangement of the Fab in three independent crystal forms has been determined by molecular replacement and refinement of the structures is in progress. The genes for the variable domain of the Fab have been cloned, sequenced and the primary amino acid sequence deduced. The three-dimensional structure will aid in an understanding of the mode by which this antibody recognizes and prevents transmission of the parasite. The system presents unique opportunities to understand neutralization from the comparison between the bound forms of differently constrained cyclic peptides and the linear counterparts and from the comparison of the free and antigen-bound MAb. The study is being extended to include the structure determination of Pfs25 and its complex with MAb 4B7.