If you want to see and print an even nicer version of this picture, take this link: asp18-2
Our labs first got interested in lipid binding proteins (LBPs) through their work on peculiar and novel forms they found from nematodes [see J.Biol.Chem.(1995) 270, 19277-19281; J.Biol.Chem.(1997) 272, 9933-9941, 272, 29442-29453; and Biochemistry (1995) 34, 6700-6710 for details]. Since then Malcolm got interested in LBPs from other sources, dragging Alan along kicking and screaming, and embroiling Jeremy Beauchamp and his molecular modeling skills and Andy Freer for his crystal gazing along the way, with lots of biochemical help from Lindsay McDermott. Maybe we got interested in the following selection of proteins because anything is worth doing instead of admin., or because exploring LBPs in all their variety and splendour might give us a clue as to what the nematode LBPs might be for (particularly in parasites) and thereby nudge towards our personal enlightenment, or once we have learned a neat set of tricks to look at these proteins we can't kick the habit and it seems to be a nice little earner.
Anyway, below is a series of pretty molecular models of proteins that we have been working on from a variety of strange sources with a few illuminating comments. There are no crystal structures of any of these proteins (yet) and the models were produced through SwissModel and CHARm refined. Malcolm did the initial modeling (despite being a zoologist), Jeremy did some further refinement where necessary and created the illustrations and Alan merely grumbled that modeling is all artifact anyway.
| As-p18. This is a fatty acid binding protein from Ascaris
suum, the large roundworm of pigs which has a close relative which
infects about a quarter of mankind. This protein was found by Baisong Mei
an Rick Komuniecki (Toledo, Ohio) in the fluid surrounding Ascaris
embryos in their eggs. We were sent some recombinant protein to play with
and found that As-p18 binds fatty acids but not retinol. We suspect that
its ligand in the egg may have something to do with the extraordinary environmental
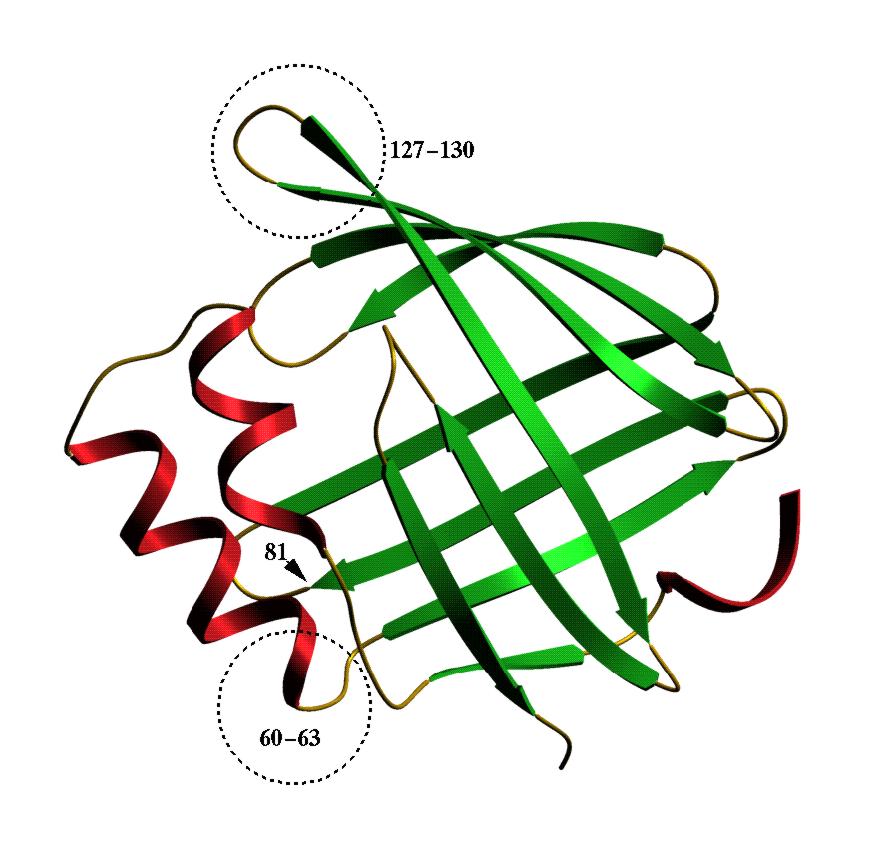
resistance Ascaris eggs exhibit. This model shows that As-p18 is
a member of the family of intracellular fatty acid binding proteins. It
does, however, have some unusual structural features which are indicated.
If you want to see and print an even nicer version of this picture, take this link: asp18-2 |
 |
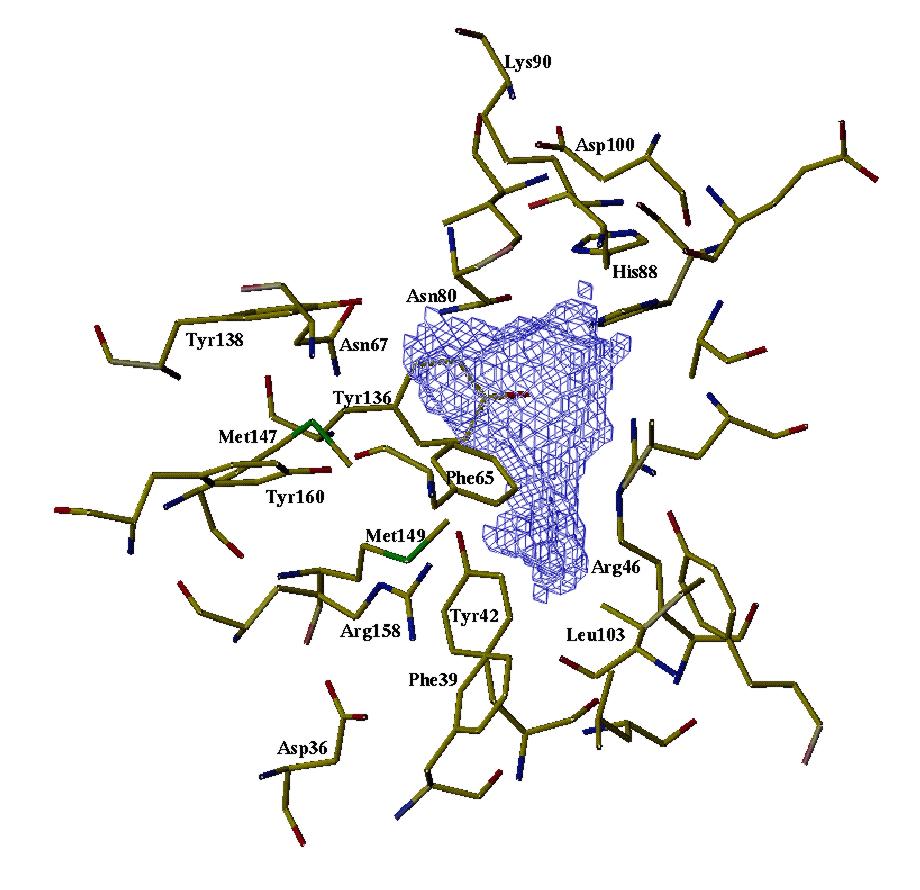 |
As-p18 binding site. This shows the cavity within As-p18 where the fatty acids presumably bind, and was produced by rattling a cyber water molecule around inside the above structure to get some idea of the shape and size of the binding site. It appears to be lined with hydrophobic amino acid side chains and opportunities for hydrogen bonding with ligand. For further details of As-p18, see Journal of Biological Chemistry (1997) 272, 9933-9941. If you want to see and print an even nicer version of this picture, take this link: asp18-1 |
| Bt6. This is a fatty acid binding protein from tropical house
dust mites (Blomia tropicalis) brought to our attention by Leonardo
Puerta of the University of Cartagena, Colombia. It seems that some people
are very allergic to this protein, so the more we know about it the better.
Leonardo came over to look at the binding properties of the protein and
found that even though it looks like a regular member of the FABP etc family,
it does not bind retinol or any of the fluorescently-tagged fatty acids
which we usually use. It does bind the naturally fluorescent fatty acid
parinaric acid, however, which some lucky b****** has to go to Tonga to
collect the nuts from which it is isolated. Anyway, this is the first FABP
described from arachnids (mites, spiders, scorpions), so a pretty stamp
for the album.
If you want to see and print an even nicer version of this picture, take this link: mite.gif |
 |
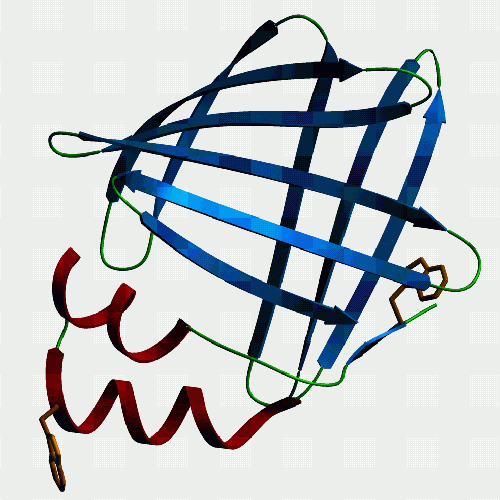
| F10. This is a fatty acid binding protein from the human blood
fluke Schistosoma japonicum which we have been working on with Julie
Scott and Don McManus at the Queensland Institute for Medical Research.
It binds fatty acids, but not retinol, and models just fine as a member
of the intracellular lipid binding protein family. Julie found a mutant
version with bits of the helices missing, and this is non-functional. The
homologue of this protein in another blood fluke (Schistosoma mansoni)
is Sm14 which is under investigation as a protective antigen for vaccination
- curiously, it seems to immunise sheep against liver fluke better than
it does humans against schistosomes. Funny old world ...
If you want to see and print an even nicer version of this picture, take this link: Jeremy's ftp-2 |
 |
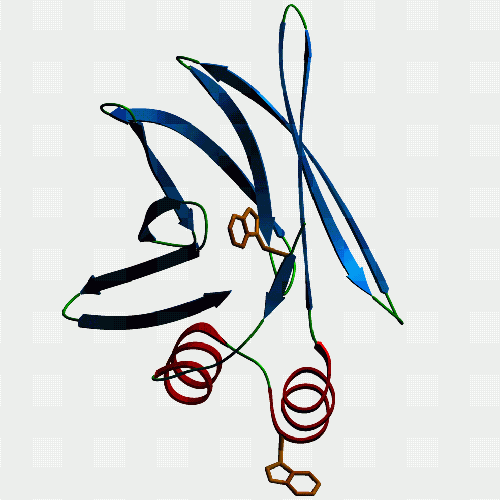 |
F10 again, but this time from a different angle to show off the strangely protruding tryptophan. Malcolm thinks that this is really significant but can't persuade Alan that investigating this will reveal the mysteries of existence and the natural world better than his devotion to denatured lysozyme (which we all make when we boil an egg, but otherwise don't give a toss about - unless you are mature enough to worry about aggregation of misfolded proteins, prion diseases, Alzheimer's, and the like... [ed.]). Anyway, according to our fluorescence measurements, the tryptophan is indeed protruding as the model predicts. Curiously, only FABPs which have been classified as exchanging fatty acids by collision with membranes rather than by diffusion of ligand have a bulky hydrophobic in this position. Interesting connection, but nobody else seems to care. So, is it involved in the protein's interaction with membranes or a membrane transport protein? If you want to see and print an even nicer version of this picture, take this link: Jeremy's ftp-1 |
| Horse p19. The only lipocalin in our gallery, and it comes from
somewhere not exactly the first place lipid binding protein people might
look - the endometrium of horses. The interesting thing is that horses
have delayed implantation of the conceptus, and p19 is only produced by
the endometrium during the time before implantation occurs, and the protein
seems to associate with the capsule which surrounds the conceptus before
it implants. So, p19 might be necessary to the conceptus's survival during
this crucial period, and is under study by Francesca Stewart's group (Babraham,
Cambridge) for this reason. We found that it binds retinol and fatty acids.
If you want to see and print an even nicer version of this picture, take this link: Jeremy's P19-1 |
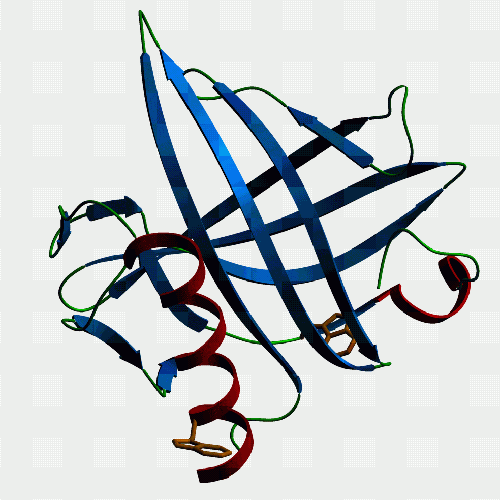 |
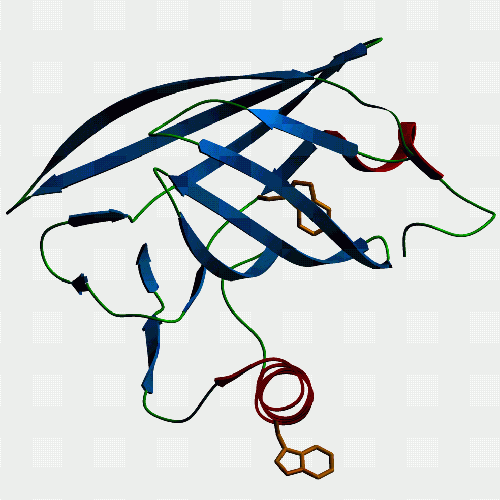 |
Another view of p19 to show that it too has a tryptophan protruding from its single helix, as modeled here, and confirmed by fluorescence measurements. Other lipocalins such as bovine lactoglobulin and human plasma retinol binding protein don't have a hydrophobic in this position and they do not associate with membranes in the same way as p19 seems to. So, ... If you want to see and print an even nicer version of these pictures, take this link: Jeremy's P19-2 |