Dr DAVID S. RYCROFT
Structural elucidation of natural products using NMR spectroscopy
![[Glasgow University Crest]](http://www.gla.ac.uk/graphics/shield_and_logotype_right.gif)
Dr DAVID S. RYCROFTStructural elucidation of natural products using NMR spectroscopy |
![[Glasgow University Crest]](http://www.gla.ac.uk/graphics/shield_and_logotype_right.gif)
|
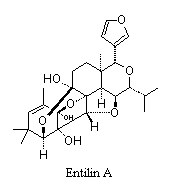 Many compounds with interesting and unusual structures have been isolated from plants used in native systems of medicine.
Many compounds with interesting and unusual structures have been isolated from plants used in native systems of medicine.