Department of Chemistry
University of Glasgow
Chemistry with Medicinal Chemistry 4H
CLASS HANDBOOK
1999 - 2000
Course Heads: Drs Peter H McCabe, Richard C Hartley
and Frances C Boyle
Course Secretary: Miss Tracy Young
CHEMISTRY WITH MEDICINAL CHEMISTRY 4H
Session 1999-2000
The class heads are:
Dr P H McCabe (Chemistry) Rm A5-11 Joseph Black Building
Tel 0141 330 4426 (direct)
E-mail P.McCabe@chem.gla.ac.uk
Dr R C Hartley (Chemistry) Rm C4-08c
Tel 0141 330 4398
E-mail R.Hartley@chem.gla.ac.uk
Dr F C Boyle (IBLS) Rm 345 West Medical Building
Tel 0141 330 5475 (direct)
E-mail F.Boyle@bio.gla.ac.uk
LECTURES
Compulsory Lectures
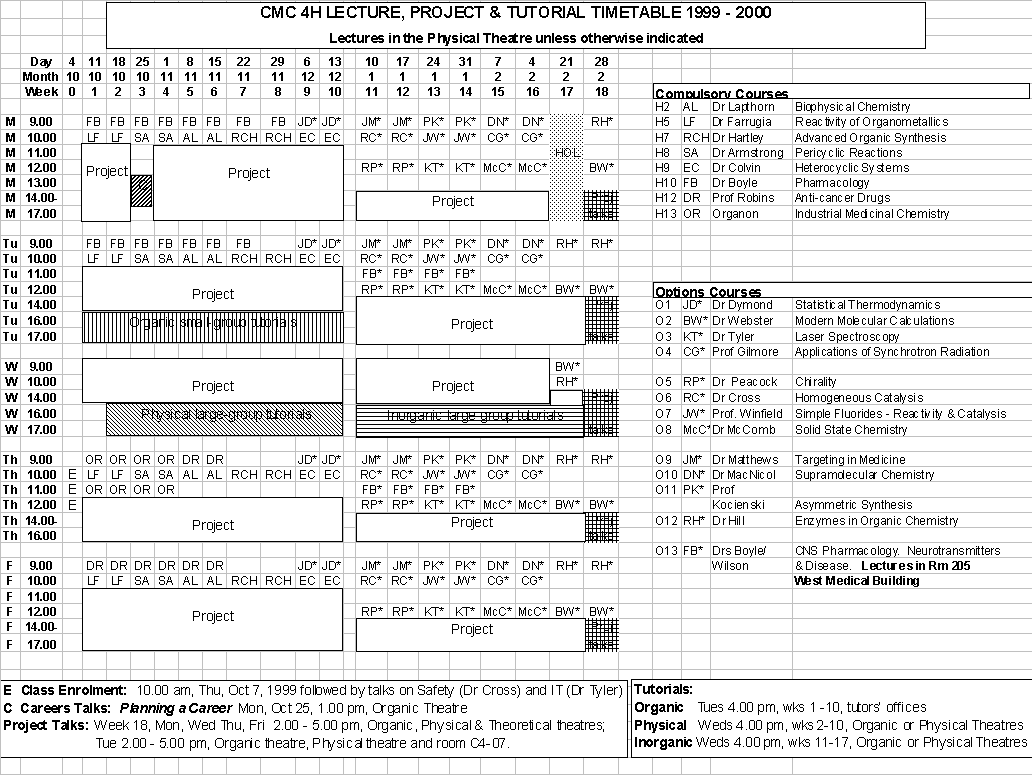
The eight compulsory lecture courses are given during weeks 1 - 10.Option Courses CMC 4H students should take the CNS Pharmacology option and four others.
Attendance at Alchemist Club and local RSC meetings, held on Thursdays at 4 pm and at the Irvine Review Lectures, held at the University of St Andrews in April, 2000, is recommended.
Lectures in Pharmacology The pharmacology lectures are given in a style which chemistry students may find unfamiliar. The material is presented more descriptively. It will not usually be possible to copy down every spoken word. Students are advised to try to understand the lecture content at the time of presentation and to make a summary of the concept/theory in note form rather than in full sentences. They can also include relevant examples or annotated diagrams. Notes should be amplified later by referring to lecture handouts or textbooks. Past examination questions (reprints available from the Alchemist Club) indicate the breadth/depth of understanding required. Some Biology 2 material will be covered for the benefit of CMC 4H students who did not previously study Biology 2. Students who feel they have already seen this material should not absent themselves from lectures but should regard them as an opportunity for revision.
TUTORIALS
Inorganic
Term 2 Wednesdays 4.00 pm, weeks 11-17, Organic or Physical Lecture Theatres.Organic Term 1 Tuesdays 4.00 pm, weeks 1-10, tutors’ offices.
Physical Term 1 Wednesdays 4.00 pm, weeks 2-10, Organic or Physical Lecture Theatres.
EXAMINATIONS
The degree examinations consist of a research project and five written examination papers (six for M.Sci. students). There is also a third year carry-over mark. Two of the written papers are each of 1.5 hours duration. All other written papers are of three hours duration.
The research project will be assessed on the basis of the thesis (40%), the work performed during the project (40%), and an oral examination on the contents of the thesis (20%).
All students must be available for oral examination by the external examiners on Tuesday, 13th June, 2000. This is an integral part of the degree examination.
Towards the end of second term, Dr Colvin will advise the class on the format of the degree examinations.

ILLNESS AND ABSENCE FROM CLASSES
If you are unable to attend classes, please consult Dr McCabe or, in his absence, Dr Hartley, as soon as possible to explain the reasons for your absence and to decide if a letter or a self-certificate or a medical certificate should be submitted. The particular situations which require submission of each certification are set out in the booklet "The Registry. A Student Guide" 1999-2000, available from the Faculty of Science office, level 3, Boyd Orr building. Self-certificate forms may be obtained from advisers of studies or from the Faculty of Science office.
If you believe that your performance in the course has been adversely affected for reasons which you wish to draw to the attention of the Board of Examiners, it is essential that you write to Dr McCabe or, in his absence, Dr Hartley, to inform him of the circumstances.
CAREERS TALKS AND DISCUSSIONS
Careers for Chemists:
On Monday, October 25, 1999 at 1.00 pm in the Organic Lecture Theatre representatives of industry, the Department of Chemistry and the University Careers Service will discuss aspects of career planning. These talks will be followed by informal discussion.During first term, Professor Winfield will arrange individual interviews for all final-year students to discuss their choices of future career.
POLICY ON SUMMATIVE ASSESSMENT
All feedback on coursework used in assessment, including mid-year class exam/class test marks and laboratory grades, is strictly provisional for your guidance only and is subject to ratification by the Board of Examiners and external examiners at the end of the academic year. You must retain all copies of assessed work (lab notebooks, exam scripts, etc.) and have them available for inspection by the examiners if requested at the end of the year. You will be given reasonable advanced warning should this be required.
PLAGIARISM
Degrees from Glasgow University recognise personal achievement. Copying is academic fraud and is a serious offence against University discipline. Discovery of copied work submitted for assessment (including lab reports, essays, examination scripts and research projects/theses) will result in credit being withheld. Plagiarism is the submission of someone else’s work as one’s own without acknowledgment. If you legitimately use/quote someone else’s work - words, ideas, data - you must acknowledge this by literature references and/or quotation marks. Where students are required to work in groups, data will necessarily be shared but each student should prepare an individual report.
STUDENT PROGRESS
Your performance of class work will be considered satisfactory only if you:
(a) regularly attend lectures and tutorials
(b) carry out a research project in weeks 2-17 following the timetable given below
(c) give a short oral presentation on your project in week 18 and
(d) provide the Chemistry Department with one copy of a thesis on your project by the first day of the third term.
During weeks 2 - 17 you are expected to devote about 20 hours per week to your project.
FOURTH YEAR PROJECT TIMETABLE
/overFOURTH YEAR PROJECT TIMETABLE
Dr Peacock is in overall charge of fourth year projects.
Week 1 Project supervisors announced to class.
Students contact supervisors and agree on projects.
Supervisors give students two copies of a synopsis of the project including a title and leading references.
One copy of the project synopsis should be given to Dr Peacock.
Week 2 Practical work begins from the start of week 2. A COSHH form must be completed, signed by the supervisor and given to Ms Lesley Bell (Room A4-42). If the nature of the research changes during the project new COSHH forms must be completed.
Week 4 Project assessors announced. Students contact assessors.
Week 10 Students give their thesis introduction to the supervisor. The supervisor will return the introduction in the first week of term 2.
The Introduction should be around 5 -10 pages, word processed, and include a full list of references.
Week 17 Friday: Last day of practical work.
Week 18 Thesis talks (15 mins - not assessed).
Week 20 Friday: Final draft of thesis approved by supervisor.
Week 21 Monday: Last day for submission of theses. One copy to be supplied to Mr R Munro - he will make a copy (or copies) for the Department and bind and return the original.
Weeks 22-23 Oral examinations. These will be conducted by the assessor and a second member of staff. The supervisor will not be present.
SAFETY
The Department of Chemistry Safety Committee has issued the following guidelines.
1. Experimental work should normally not start before 9.00 am and should normally finish by 5.00 pm.
2. Practical work for short periods outwith these hours must be approved by the supervisor and the usual rules of late working will apply. If the supervisor has to leave before experimental work is complete, he or she must give written permission to the student to continue work and a designated proxy (academic, post-doctoral or senior technical staff) must be present in the building.
3. Access to IT equipment will be available only when security staff are on duty.
GUIDELINES FOR WRITING AND PRESENTING A THESIS
The thesis counts for 40% of the marks assigned to the project and is the only tangible result of the sixteen weeks of reasearch which can be shown to the External Examiners. It is therefore important that you devote sufficient time to your research and do not let yourself down by a badly written or produced thesis.
Technical Points
The thesis should be word processed (the Department now has an adequate number of PC’s with WORD 7 installed).
The font should be clear. Fonts normally used are Times New Roman or Arial (usually 10, 11 or 12 point). This document is written in Arial 10 pt with main headings in 12 pt bold.
The thesis should use 1.5 line spacing and have a reasonable margin on the left hand side to allow for binding. Margins of 3.17 cm left and right and 2.54 cm top and bottom are acceptable.
Pages should be numbered consecutively, as should diagrams and spectra. Since the word processor will do the numbering for you, it is easier if you do not include whole page diagrams or spectra in the page numbering but this is a matter of choice.
Chemical structures can be drawn using ChemDraw and copied into WORD 7. On the other hand, there is nothing wrong with photocopied figures cut and pasted manually provided that the result looks neat and clear.
References
The referencing of previous work is very important (see "Plagiarism" above) and is frequently badly done. The format shown in the following examples is that employed by the Royal Society of Chemistry. It should be used unless your supervisor suggests an alternative.
1. Journal articles: Journal in italics, year, volume no in bold, page no, eg
I. A. Fallis, L. J. Farrugia, N. M. Macdonald and R. D. Peacock, J. Chem. Soc. Dalton Trans., 1989, 931.
P. R. Mallinson and K.W. Muir, J. Appl. Crystallogr., 1985, 18, 51.
Other possibilities are: unpublished work, in press, personal communication.
2. Books: Title in italics, publisher, place, year, vol no, page if necessary, eg
International Tables for X-ray Crystallography, Kynoch Press, Birmingham, 1974, vol 4.
3. Theses:
N. M. Macdonald, Ph.D. thesis, University of Glasgow, 1994.
P.A. Lovatt, B.Sc. thesis, University of Glasgow, 1993.
Thesis Sections
The thesis should contain
Title page
Acknowledgements
Contents page (with page numbers)
A one-page Abstract
Introduction
Experimental Section,
Results and Discussion (or Results and Discussion as separate sections)
Conclusions
References
In some areas of chemistry, the Experimental Section may be placed after the Conclusions.
The Introduction should set the work in context, review previous work (fully referenced), describe any techniques or theories with which you were unfamiliar when you began the research, and describe what you intended to do.
The Experimental Section should give full experimental details of all reactions or experiments carried out. It is particularly important to indicate which are literature preparations and which are novel. If a literature preparation is reported it is important to note if you modified it or if it behaved in an unexpected way. New compounds should be characterised as fully as possible and it is a good idea to include their spectra.
The Discussion is extremely important and is often where students do not do themselves justice. A project where absolutely nothing has worked can be made interesting by discussing WHY things went wrong. In any case, the discussion is often where you show how much of the project you understand!
The Conclusions should summarise your experimental findings in context and suggest how the project could be extended in the future.
The Abstract will be similar to but more concise than the Conclusions. It should state clearly and incisively what you achieved - it is the first thing an examiner will read and should encourage him or her to read the rest of the thesis!
You must spell-check written work but note that abbreviations, acronyms and all chemical names will tediously appear as wrongly spelled words.
CHEMISTRY DEPARTMENT LIBRARY
The library holds up-to-date editions of textbooks, which are recommended below for consultation, and a limited supply of course handouts/tutorial sheets. During week 2, the librarian, Mrs Denise Currie, will run, in the library, one-hour instruction sessions entitled "An Introduction to the Chemical Literature".
CHEMISTRY WITH MEDICINAL CHEMISTRY 4H: RECOMMENDED TEXTBOOKS FOR SESSION 1999-2000
(A) ESSENTIAL PURCHASES
Molecular models: Framework Molecular Model Student Set,
Prentice Hall, ISBN 0-13-330076-5, normally £23.95 but the publishers have agreed a discounted price of £12. OROrbit Molecular Building System: Organic and Inorganic Chemistry Individual Set, Cochrane, £13.04.
Inorganic Chemistry, Third Edition, D F Shriver, P W Atkins and C H Langford, Oxford University Press, 1999, £21.99.
Spectroscopic Methods in Organic Chemistry, Fifth Edition Revised, D H Williams and I Fleming, McGraw-Hill, £17.99.
Organic Synthesis: The Disconnection Approach, S Warren, John Wiley & Sons, £18.99.
Organic Chemistry, Second Edition, P A Bruice, Prentice Hall, £26.95.
Physical Chemistry, Second Edition, R A Alberty and R J Silbey, John Wiley, £24.95.
Chemical Bonding Theory, B C Webster, Blackwell Scientific, Oxford, 1990, £24.95
(B) RECOMMENDED READING
Basic Solid State Chemistry, Second Edition, A R West, John Wiley, £22.95.
Structural Methods in Inorganic Chemistry, Second Edition, E A V Ebsworth, D W H Rankin and S Craddock, Blackwell. Currently out-of-print but second hand copies may be available.
Particularly useful for laboratory and tutorial work and helpful in problem solving.
The Mechanisms of Reactions at Transition Metal Sites, R A Henderson, Oxford Science Publications, £5.99.
Pericyclic Reactions, I Fleming, Oxford Chemistry Primers, £5.99. An alternative is: Frontier Orbitals and Organic Chemical Reactions, I Fleming, Wiley, 1976.
Workbook for Organic Synthesis: The Disconnection Approach, S Warren, Wiley, £16.99.
Reactive Intermediates, C J Moody and G H Whitham, Oxford Chemistry Primers, £5.99.
Stereoselectivity in Organic Synthesis, G Procter, Oxford Chemistry Primers, £5.99.
Stereoelectronic Effects, A J Kirby, Oxford Chemistry Primers, £5.99.
Chemical Aspects of Biosynthesis, J Mann, Oxford Chemistry Primers, £5.99.
An Introduction to Medicinal Chemistry, G L Patrick, Oxford University Press, £18.50, (CMC only).
Aromatic Heterocylic Chemistry, D T Davies, Oxford Chemistry Primers, £5.99.
Group Theory for Chemists, G Davidson, Macmillan.
Crystal Structure Determination, W Clegg, Oxford Chemistry Primers, £5.99.
Computational Chemistry, G H Grant and W G Richards, Oxford Chemistry Primers, £5.99.
Beginning Mathematics for Chemistry, S K Scott, OUP, £9.99. Worth buying if you feel that your mathematical skills need to be improved.
NOTE: ALL PRICES ARE SUBJECT TO CHANGE BY PUBLISHERS AT ANY TIME.
(C) REFERENCE BOOKS HELD IN THE CHEMISTRY BRANCH LIBRARY
INORGANIC CHEMISTRY
Advanced Inorganic Chemistry, Fifth Edition, F A Cotton and G Wilkinson, John Wiley.
Chemistry of the Elements, N N Greenwood and A Earnshaw, Pergamon.
More of a reference book than a textbook, but contains factual information, particularly for main group elements, which is more complete than in Cotton and Wilkinson.
Some Thermodynamic Aspects of Inorganic Chemistry, Second Edition, D A Johnson, Cambridge U.P. (Out of Print)
Inorganic Chemistry, Third Edition, A G Sharpe, Longman.
Orbitals, Terms and States, M Gerloch, John Wiley, out of print.
A small book which should help to clarify difficulties in understanding the nature of orbitals, terms and states. Relevant to many courses in Inorganic and Physical Chemistry.
The Elements, Their Origin, Abundance and Distribution, P A Cox, O.U.P.
Useful background and revision materials for Radiochemistry courses.
A Guide to Modern Inorganic Chemistry, S M Owen and A T Brooker, Longman.
Sets out to answer the questions that are asked most often by students. A revision aid.
Heterogeneous Catalysis. Principles and Applications, G C Bond, O.U.P.
ORGANIC CHEMISTRY
A Guidebook to Mechanism in Organic Chemistry, Sixth Edition, P Sykes, Longmans.
Some Modern Methods of Organic Synthesis, Third Edition, W Carruthers, Cambridge U.P.
Guidebook to Organic Synthesis, Second Edition, R K Mackie, D M Smith and R A Aitken, Longman.
Advanced Organic Chemistry - Reactions, Mechanisms and Structure, Fourth Edition, J March, John Wiley. The definitive reference work which is an excellent and complete source of references.
Secondary Metabolism, Second Edition, J Mann, Carendon Press.
Medicinal Chemistry, C R Ganellin and S M Roberts, Academic Press.
Physical Organic Chemistry, N S Isaacs, Longman.
Heterocyclic Chemistry, Third Edition, T L Gilchrist, Longman.
Heterocycles in Life and Society, A F Pozharskii, A T Soldatov and A R Katritzky, Wiley.
Non-Benzenoid Conjugated Carbocyclic Compounds, D Lloyd, Elsevier. (Out of print)
Molecular Biology of the Gene, Volumes 1, & 2 Fourth Edition, J D Watson et al., Benjamin//Cummings.
Organic Chemistry - A Guide to Common Themes, T Kitson, Edward Arnold.
A very readable overview of concepts and reaction types.
PHYSICAL CHEMISTRY
Beginning Group Theory for Chemistry, P H Walton, OUP.
Chemical Applications of Group Theory, Third Edition, F A Cotton, John Wiley.
Tables for Group Theory, P W Atkins, M S Child and C S G Phillips, O.U.P.
Molecular Quantum Mechanics, Second Edition, P W Atkins, O.U.P.
Modern Spectroscopy, Third Edition, J M Hollas, John Wiley.
Fundamentals of Molecular Spectroscopy, Fourth Edition, C N Banwell, McGraw-Hill.
Crystal Structure Analysis: A Primer, Second Edition, J P Glusker and K N Trueblood, O.U.P.
Symmetry and Structure, S F A Kettle, John Wiley.
Biophysical Chemistry, C R Cantor and P R Schimmel, W H Freeman & Co.
Physical Biochemistry, D Freifelder, W H Freeman & Co.
Enzyme Structure and Mechanism, A Fersht, W H Freeman & Co.
Protein Structure - a practical approach, T E Creighton, IRL Press.
Introduction to Protein Structure, C Branden and J Tooze, Garland Publishing.
Physical Chemistry, Sixth Edition, P W Atkins, OUP.
Molecular Modelling. Principles & Applications, A R Leach, Addison Wesley Longman, 1996, ISBN 0-582-23933-8, £35. Particularly relevant to Dr White’s course on Computational Chemistry.
PHARMACOLOGY
Pharmacology, Fourth Edition, Rang, Dale & Ritter, Churchhill-Livingstone.
Introduction to Drug Metabolism, Second Edition, G G Gibson & P G Skett, Chapman Hall.
Neuropharmacology, T W Stone, Freeman.
(D) REFERENCE BOOKS HELD IN THE MAIN LIBRARY
Biology, Fifth Edition, N A Campbell
PHARMACOLOGY
Medical Pharmacology at a Glance, Third Edition, Addison Wesley Longman
Pharmacology, Second Edition, M J Mycek, R A Harvey and P C Champe, Lippencott-Raven
AIMS AND OBJECTIVES
The aims and objectives for the two options courses Applications of Synchrotron Radiation (Prof. Gilmore) and Asymmetric Synthesis (Prof. Kocienski) will be distributed during the lecture courses. Aims and objectives for other courses are given in the following section of the handbook.
Title: Pharmacology
Lecturer(s): Dr F C Boyle
Aims: To explain the general processes which affect drug action and to examine how these processes determine the quantitative and, to some extent, the qualitative effect of drugs. In particular, the aim is to explain current views on the drug-receptor interaction and generally how drugs can be used to interfere specifically with endogenous mechanisms to produce useful therapeutic effects. In addition, the methods used to study drug action and the way new drugs are designed and developed are reviewed.
Objectives: Students should:
1. Understand the nature of Pharmacology and the methods used to study drug action.
2. Appreciate the wide range of processes which determine the rate of onset, duration and intensity of drug effects.
3. Understand the mechanisms by which drugs produce their effects, particularly by acting on drug receptors and thereby modifying key homeostatic mechanisms.
4. Understand the concepts of drug receptors, agonists, partial agonists, competitive and insurmountable antagonists, pAx values, spare receptors, affinity and efficacy, pD2 values, Schild analysis, second messenger systems.
5. Understand the complexity of the mechanisms by which drugs may interact in the body and the mechanisms which underlie the toxic effects of drugs.
6. Appreciate that the capacity to produce new, more potent, selective and less toxic drugs depends on understanding the relationship between chemical structure and drug action.
7. Appreciate that the capacity to produce new, efficacious drugs also depends on understanding, at the molecular level, the homeostatic mechanisms which control normal functioning and which are deranged in disease.
Outline:. The series of lectures establishes the principles and concepts upon which pharmacology is based. Students are introduced to the definition and source of drugs and the techniques used to study drug action. The complex sequence of events following the administration of a drug is examined and this sequence is used to consider the administration, adsorption, distribution of drugs, the mechanisms underlying the interaction of drugs with their receptors, their bio- transformation and excretion. The many factors which affect drug action are examined. Drug development and the screening of new drugs are reviewed. The lectures on drug/receptor interaction outline the development of the concept of receptors on which drugs act, the interaction of agonistic and antagonistic drugs with receptors and the mechanisms of transduction by which receptor activation leads to changes in cell activity.
Title: Chemistry and Pharmacology of Anti-cancer Drugs
Lecturer(s): Prof. D J Robins, Dr A D Lewis (Quintiles)
Aims: To discuss the main agents used in the treatment of cancer in terms of synthesis, chemistry, metabolism and mode of action.
Objectives:
1. Know about abnormal cell growth and its causes and possible treatments.
2. Recognise different types of alkylating agents, their synthesis, mechanism of action and pharmacology.
3. Understand the concept of antimetabolites with synthesis and mode of action of examples such as 5-fluorouracil and methotrexate.
4. Understand hypoxia in tumours and selectivity of action achieved by bioreductive agents containing quinones or N-oxides; know about the mode of activation and action of bioreductive agents such as mitiomycin C.
5. Know about natural products used as anticancer agents, particularly doxorubicin with partial synthesis and mode of action; topoisomerase inhibitors and microtubule inhibitors.
6. Understand the importance of growth factors and inhibition of signalling processes by drugs as new targets.
7. Know about the synthesis, spectroscopy and instability of tyrosine kinase inhibitors and erbstatin.
Outlines:
Cancer Biology: a disease of abnormal growth and cellular proliferation; causes and methods of treatment; development of anti-cancer drugs; importance of selectivity; classes of anti-cancer agents including alkylating agents, antimetabolites, bioreducible compounds, natural products and compounds which interfere with cell signalling processes; pharmacokinetics and mechanism of action; drug design and drug resistance; drug development based on mechanism of action.
Chemistry of anticancer drugs: alkylating agents including nitrogen mustards, cyclophosphamide, nitrosourea (synthesis, action and interaction with DNA); antimetabolites including 5-fluorouracil and methotrexate (synthesis and mechanism of action); natural products such as doxorubicin and taxol (partial synthesis, spectroscopic characterisation); tyrosine kinase inhibitors including active compounds made in this Department (synthesis and spectroscopic characterisation).
Title: Industrial Medicinal Chemistry
Lecturer(s): Dr. D. Rees and Dr. N. Hamilton (Organon)
Aims: To study case histories of the development of selected important medicinal compounds from the perspective of industrial researchers who are familiar with these therapeutics areas
Objectives:
1. Know about the discovery and chemical modification of lead compounds and structure-activity relationships.
2. Be able to describe how morphine was modified to produce useful analgesics.
3. Know about the synthesis and biological activity of kappa-selective opioids.
4. Be able to discuss the importance of combinatorial chemistry.
5. Know about common routes of administration of drugs.
6. Know about some common metabolic pathways and how they may be exploited in the design of prodrugs and soft drugs.
7. Be able to discuss the therapeutic potential for GABA A receptor modulators with particular reference to general anaesthetics including steroids.
Outline: Relationships between physical properties of organic compounds and biological activity, structure-activity relationships, discovery and modification of lead compounds, drug design and combinatorial chemistry. Clinical use and limitations of morphine, chemical modification, agonist vs. antagonist activity, irreversible ligands and simplified morphine derivatives. Multiple opioid receptors including kappa, development of initial leads by synthesis, importance of stereo- chemistry on biological activity. Need for combinatorial methods, solid phase peptide synthesis, peptide libraries, solid phase synthesis of benzodiazepines. Description of routes of administration and their importance in optimising a drug effect. The concepts of prodrugs and soft drugs and examples with common metabolic pathways. Synthesis and biological activity of a novel steroidal intra- venous anaesthetic drug.
Title: Biophysical Chemistry
Lecturer(s): Dr A Lapthorn
Aims:
To develop an awareness of some methods used to study the physical properties of biological molecules.Objectives:
1. Discuss the variety of biological molecules and describe the properties of amino acids, peptides and proteins.
2. Discuss methods and techniques for the determination of molecular weight and amino acid sequencing.
3. Discuss the study of protein conformation using spectroscopic and diffraction techniques.
4. Discuss the binding of ligands to macromolecules, enzyme kinetics and molecular dynamics.
5. Use information provided to discuss topics concerning the structure, function, and characterisation of proteins and other biological macromolecules.
Outline:
This course of lectures will introduce some physical chemistry aspects of biological molecules. The nature of biological molecules; the properties of amino acids, peptides and proteins; methods for establishing molecular weight and physical properties; determination of the composition of proteins: amino acid analysis, amino acid sequencing; sequencing at the DNA level; UV absorption spectroscopy: effects of pH, polarity, etc.; applications; fluorescence spectroscopy: effects of pH, temperature, ligand binding, etc.; applications; circular dichroism: the measurement of protein secondary structure content; NMR: use of 2D-NMR to derive protein structure; use of 31P-NMR to measure effects in whole cell. Diffraction methods: differences between protein and small molecule crystallography; examples of protein structures; chymotrypsin, trypsin and elastase specificities; Solution scattering: low angle X-ray scattering; neutron scattering; phase contrast techniques; measurement of binding of ligands to macromolecules:
Title: Advanced Organic Chemistry
Lecturer(s): Dr R C Hartley
Aims: To introduce new methods of forming carbon-carbon bonds. To illustrate these methods with syntheses of biologically active molecules and to show the importance of organometallics in carrying out unconventional transformations.
Objectives:
1. Suggest reagents for alkylidenation of carbonyl compounds, describe the mechanisms of alkylidenation reactions and apply these reactions to synthesis.
2. Count electrons in a metal complex, recognise and explain the importance of coordinative saturation, and describe the basic types of reaction found in most catalytic cycles (association and dissociation; oxidative addition and reductive elimination; ligand to metal migration and metal to ligand migration; nucleophilic attack on ligands).
3. Describe mechanisms for palladium(0)-catalysed cross-coupling and carbonylative cross-coupling reactions (in general and for particular examples), explain any restrictions to the substrates, and apply these reactions to synthesis.
4. Suggest appropriate syntheses of organoboron, organosilicon, organosulfur, and organometallic compounds (i.e. those of lithium, magnesium, tin, zinc etc.), understand their reactivity, give mechanisms for their reactions and apply these compounds to synthesis.
5. Devise suitable syntheses of organic halides.
6. Describe mechanisms for the reactions of allylborons and allylsilanes, apply these reagents to synthesis and explain diastereoselectivity in the reactions of crotylboronates and crotylsilanes.
Outline:
Alkylidenation reactions: Wittig (revision), Julia, Peterson, Petasis and Takeda alkylidenation reactions. Synthesis of alkenes from alkynes. Fundamental organotransition metal chemistry (electron count in complexes). Pd(0) and Ni(0) catalysed cross-coupling reactions and their application to synthesis. Synthesis and use of organolithiums, Grignard reagents (revision), organozincs, organoborons, organotins, and other organometallics. Synthesis and use of organic halides. Allylborons, allylsilanes, crotylborons and crotylsilanes and the synthesis of homoallylic alcohols; Sakurai and related reactions.
Title: Pericyclic Reactions
Lecturer(s): Dr S K Armstrong
Aims:
To develop an understanding of cycloadditions and pericyclic rearrangement reactions, and of their importance in organic chemistry, based on frontier orbital interactions and the Woodward-Hoffmann Rules.
Objectives:
1.
Appreciate that the outcomes of many cycloaddition and rearrangement reactions may be understood in terms of frontier orbital interactions.2. Recall or derive the p -orbital systems of alkenes, dienes, and allyl systems, whether substituted or not.
3. Predict and rationalise the outcomes of Diels-Alder cycloadditions, including stereospecificity, regioselectivity, and stereoselectivity, in terms of primary and secondary orbital interactions.
4. Predict and rationalise the outcomes of other cycloaddition reactions, including 1,3-dipolar and [2+2] cycloadditions. Understand, recall and apply the Woodward-Hoffmann Rules as applied to cycloaddition reactions in thermal or photochemical conditions.
5. Predict and rationalise the outcomes of [3,3]-sigmatropic rearrangements, including regioselectivity and stereoselectivity, in terms of primary and secondary orbital interactions.
6. Predict and rationalise the outcomes of other rearrangements, in particular [2,3]-sigmatropic rearrangements, in terms of frontier orbital interactions and the Woodward-Hoffmann Rules.
7. Apply the above understanding to examples published in the chemical literature.
Outline:
Introduction Shape of bonding and anti-bonding s and p orbitals; extended p systems; energy levels; orbitals in control of familiar reactions such as SN2. Diels-Alder Cycloaddition The basic reaction: a reminder. Orbitals involved and their implications for transition state geometry. Stereospecificity with respect to diene and dienophile. Substituted dienes and dienophiles and their orbitals. Orbital energy and its effect on reaction rate. Regioselectivity determined by orbital coefficients. Stereoselectivity (exo/endo) governed by secondary orbital interactions. Intramolecular reactions. Other Cycloadditions 1,3-Dipolar cycloadditions. [2+2] Cycloadditions. Woodward-Hoffmann Rules as applied to cycloadditions. Pericyclic Rearrangements [3,3]- and [2,3]-sigmatropic rearrangements, including Claisen and related rearrangements, and rearrangements involving sulfur and selenium. Orbital involvement; chair-shaped transition states; stereochemical control.
Title: Heterocyclic Systems
Lecturer(s): Dr E W Colvin
Aims: To present the essential features of heterocyclic chemistry, dealing with the synthesis of selected ring systems and their varied reactivity. The main emphasis will be on unsaturated ring systems.
Objectives:
1. Understand aromaticity as it applies to unsaturated heterocycles.
2. Be aware of the synthesis of pyrroles, thiophenes and furans, including the Paal-Knorr synthesis, and understand their electrophilic substitution and anion chemistry.
3. Be aware of the synthesis of oxazoles, imidazoles and thiazoles, and understand their electrophilic and nucleophilic substitution and anion chemistry.
4. Be aware of the synthesis of quinolines and isoquinolines, including the Skraup and Bischler-Napieralski syntheses, and understand their electrophilic and nucleophilic substitution and anion chemistry.
5. Be aware of the synthesis of indoles, including the Fischer synthesis, and understand their electrophilic substitution and anion chemistry.
6. If time permits, coumarins, chromones and pyrimidines will also be discussed.
Title: Reactivity of Transition Metal Organometallic Compounds
Lecturer(s):
Dr L J FarrugiaAims: To understand some of the basic reactions of organic ligands which are coordinated to transition metals and how certain ligands may be stabilised upon coordination.
Objectives:
1. Understand the bonding in, and types of compounds formed by the cyclopentadienyl ligand; know about the electrophilic substitution and metallation reactions of ferrocene.
2. Know some examples of the differing substitution rates in dienyl versus cyclopentadienyl compounds and the reasons and evidence for ring slippage.
3. Understand how some unstable and non-existent molecules are stabilised by coordination to transition metals; know some examples such as cyclobutadiene and trimethylene methane.
4. Understand how the bonding within butadiene is changed by coordination to Fe(CO)3 and be able to provide evidence for this; know the Green/Mingos/Davis rules for deciding the site of nucleophilic attack at coordinated polyenes and be able to use these rules in concrete examples.
5. Know how both Fischer and Schrock carbenes are made and the reasons for their different reactivities.
Outline:
Metallocenes and revision of bonding therein; versatility of the C5H5 ligand - occurrence in both high and low oxidation state compounds; half sandwich compounds, bent metallocenes and triple decker sandwiches; structure and syntheses of main group analogues of Cp such as P5, As5, C4H4P, C4H4BMe, etc; aromaticity of ferrocene and electrophilic substitution reactions.
Stabilisation of unstable molecules such as cyclobutadiene, trimethylene methane, benzyne and Bi2 by co-ordination to transition metals.
Hückel approach to binding in butadiene and how this is affected by co-ordination to Fe(CO)3; structural and 13 C NMR evidence; nucleophilic attack at co-ordinated polyenes; the Green/Mingos/Davis rules for determining the site of nucleophilic attack.
Nucleophilic attack at CO, the formation of Fischer carbenes; nucleophilic reactions of the Fischer carbene; synthesis of Shrock carbenes and the reason for their different reactivity.
Title: Statistical Thermodynamics
Lecturer(s): Dr J Dymond
Aims: To show how equilibrium thermodynamic property data for dilute gases and solids, and chemical reaction rates, can be related to properties of the individual molecules.
Objectives:
1. Derive the number of ways of distributing n indistinguishable particles among g degenerate energy states.
2. Give the corrected Boltzmann statistics for the total number of arrangements of N particles, where ni are in energy level ei which has a degeneracy gi
3. State the Boltzmann distribution law and use it to determine the relative populations of different energy levels.
4. Appreciate the meaning and importance of the molecular partition function and relate it to the total energy of a system.
5.
Factorise the molecular partition function6. Give the expression for the translational partition function and hence the contribution to the energy and heat capacity at constant volume for an ideal gas.
7. Give a statistical thermodynamic explanation for ideal gas expansion at constant temperature.
8. Appreciate the impossibility of obtaining absolute energies.
9. Give the expression for the rotational partition function, and know the meaning of the characteristic rotational temperature and the symmetry number.
10.
Give the contribution to the energy and heat capacity at constant volume from rotational motion.11. Give the expression for the vibrational partition function and know the meaning of the characteristic vibrational temperature.
12. Calculate the contribution to the energy and heat capacity at constant volume arising from vibrational motion.
13. Describe ortho- and para-states of diatomic molecules and understand the alternating intensities to be found in rotational Raman spectra.
14. Calculate enthalpy changes for reactions.
15. Account for the temperature dependence of the heat capacities of solids.
16. Relate the Boltzmann expression for entropy to the classical entropy.
17. Give the statistical thermodynamic expression for entropy in terms of energy and the molecular partition function.
18. Give the Sackur-Tetrode equation and demonstrate that the dependence of entropy for an ideal gas on T at constant V, and on V at constant T, agrees with classical results.
19.
Explain what is meant by residual entropy, and calculate its values for certain systems.20. Relate the Helmholtz and Gibbs energies to the molecular partition function.
21. Give approximate values for the translational, rotational, vibrational and electronic contributions to the molecular partition function.
22. Explain chemical equilibrium in terms of the distribution of molecules among energy levels and hence understand the molecular factors that influence the position of equilibrium.
23. Describe transition state theory and derive and expression for absolute reaction rates.
24. Calculate steric factors for reactions involving molecules of differing complexity.
Outline:
This course is designed to show how equilibrium thermodynamic data for dilute gases and solids, and chemical reaction rates, depend upon the properties of the constituent molecules.
Introduction - derivation of the numbers of ways of distributing indistinguishable particles among degenerate energy levels, corrected Boltzmann statistics and simple applications; the molecular partition function (q); dependence of the internal energy on q; factorisation of q; the contribution from translational motion and translational energy; absolute energies; rotational partition functions and the symmetry number; rotational energy and heat capacity contribution; vibrational partition function, the characteristic vibrational temperature and the contribution to the heat capacity; electronic partition function; effects of nuclear spin - ortho and para forms; relative intensities of rotational Raman spectra; enthalpy changes for chemical reactions; classical and statistical entropies.
Sackur-Tetrode equation for monatomics; residual entropy for simple diatomics and gases; free energy changes; chemical equilibrium; simple collision theory of reaction rates; activated complex theory, and examples.
Title: Laser Spectroscopy
Lecturer(s): Dr J K Tyler
Aims: This course is intended to introduce the principles of laser operation in general and the details of specific devices. Some important applications of lasers in chemistry and spectroscopy are touched on.
Objectives:
1. The nature of radiative transitions and basic laser theory.
2. Ways of achieving population inversions.
3. Optical gain and feedback, and the criteria for pulsed and continuous operation.
4. Rare gas discharge lasers, molecular infrared gas lasers, hydrogen halide chemical lasers and organic dye solution lasers.
5. Diode lasers.
6. Excimer lasers and super-radiance.
7. The laser as a spectroscopic source and as an excitation source for Raman spectroscopy.
8. Flash photolysis with lasers.
9. Multiphoton processes and infrared photochemistry.
Outline:
Radiative and non-radiative energy changes. Spontaneous and stimulated radiative processes and basic laser theory. Practical realisation of laser action. Population inversion. The ammonia maser. Optical gain and feedback. The ruby and neodymium ion lasers. Criteria for pulsed and continuous operation. Rare gas discharge lasers. Details of the He/Ne device. Molecular gas lasers operating in the infrared region exemplified by the CO2/N2 system. Hydrogen halide chemical lasers. Organic dye solution lasers.
Semiconductor levels and diode lasers. The N2 laser. Super-radiance. Excimer and exciplex lasers. The nature of laser radiation. The laser as a spectroscopic source. Tunability. The laser as an excitation source for Raman spectroscopy. Flash photolysis with lasers. Multiphoton processes. Infrared photochemistry. Laser separation of isotopes.
Title: Modern Molecular Calculations
Lecturer(s): Dr B C Webster
Aims: The aims of the course are to introduce the computer based methods which are available for the ab-initio and semi-empirical calculation of molecular properties and to review and develop some of the concepts which were introduced in third year courses in quantum mechanics and bonding theory.
Objectives:
1. Appreciate the need for and the validity of approximate calculations and relate them to the approximations inherent in measurement.
2. Appreciate calculation strategies on large computers without becoming involved in the details of the programming.
3. Approach with a more critical attitude results obtained from the use of large established computer programs.
4. Describe the general structure of calculations of Hartree Fock/Self Consistent Field (HF/SCF) wavefunctions.
5. Describe the various approximations involved in HF/SCF calculations.
6. Describe what is meant by a minimal basis set and an extended basis set and describe various commonly used basis sets of Gaussian functions matched to Slater type orbitals: STO-3G, 3-21G, 3-21G(*), 6-21G, 6-31G*, 6-31G**.
7. Explain why the number of basis functions used in a calculation is limited by available computing time.
8. Describe how molecular geometry can be optimised.
9. Describe the effects of neglecting electron correlation.
10. Describe the general structure of calculations of configuration interaction (CI) calculations.
11. Describe the various approximations involved in CI calculations.
12. Explain the need to use limited CI methods to reduce computer time.
13. Describe the trade-off between accuracy of calculation and computing time and how this is related to molecular size.
14. Explain why ab-initio methods cannot at present be applied to very large molecules and the need for semi-empirical methods.
15. Describe the basis of the main types of semi-empirical methods: NDDO, INDO and CNDO including the main approximations made and the major variants.
16. Explain the value of parameterisation in semi-empirical methods.
17. Assess the likely accuracy of properties such as equilibrium geometries, vibrational frequencies, absolute entropies, barriers to internal rotation and inversion, electronic dipole moments and bond dissociation energies calculated using ab-initio and semi-empirical methods.
18. Describe the Born-Oppenheimer approximation and its effect.
19. Write the molecular Hamiltonian operator in atomic units within the Born-Oppenheimer approximation for any molecule.
20. State the requirements for a satisfactory electron wavefunction.
21. Describe the relationship of Slater-type orbitals and Gaussian-type atomic functions to hydrogenic wavefunctions.
Outline:
Revision of some quantum mechanical concepts. The HARTREE-FOCK-ROOTHAAN method. Basis sets. Strategies for solution of HFR equations. Electron correlation. Semi-empirical techniques.
Title: Chirality
Lecturer(s): Dr R D Peacock
Aims: The aims of the course are to provide an introduction to the occurrence and importance of chirality; to explain how chiral molecules may be detected, resolved or synthesised and to give an appreciation of circular dichroism spectroscopy and its use in determining the absolute configuration of chiral molecules.
Objectives:
1. Understand the basic definitions of chirality - enantiomer, diasterioisomer, racemate. Have an appreciation of the importance of chirality in the interaction of chiral molecules with natural systems such as man.
2. Appreciate the idea of a chromophore. Know the common types of chromophore and the transitions which they undergo.
3. Understand the concept of the polarisation of an electronic transition, of plane polarised light and of plane polarised absorption spectroscopy. Understand how the polarisation of a transition can be derived by multiplying the phase of the HOMO with that of the LUMO (or higher unoccupied molecular orbital).
4. Be able to use phase multiplication to determine the polarisations of the p -> p * transition of ethene and the d -> d * transition of the Mo2 chromophore and appreciate how the method can be used to determine the polarisation (long or short axis) of the transitions of polyacenes such as substituted benzenes, naphthalene and anthracene.
5. Understand how in the twisted Mo2 chromophore there is a transient helical charge distribution during the d -> d * transition and how this charge distribution interacts differently with left and right circularly polarised light leading to circular dichroism. Appreciate how the sense of twist of the chromophore (and hence the absolute configuration) is related to the sign of the CD spectrum.
6. Understand the concept of chirally coupled (organic) chromophores. Understand how the CD spectrum of such a system is related to the chirality of the molecule. Be able to determine the chirality of a pair of coupled chromophores from the sign of its CD spectrum.
7. Understand how enantiomers can interact in homochiral or heterochiral pairs of stacks. Appreciate the molecular basis of diastereomeric interactions ("three point model").
8. Know the common methods of resolving chiral molecules.
9. Be able to describe and appreciate the molecular basis of the various methods of detecting chiral molecules - chiral chromatography, chiral NMR reagents, chiral sensors.
10. Appreciate the molecular basis of asymmetric catalysis - particularly as applied to the examples given in lectures.
Outline:
General definitions of chirality etc.; chromophores; polarisation of electronic transitions; the alkene and dimolybdenum chromophores; twisted chromophores and circular dichroism; coupled chromophores - bianthryls etc, use in determining absolute configuration; diasteriomeric interactions; resolution of enantiomers; chiral GLC, chiral HPLC, chiral NMR shift reagents, chiral sensors; asymmetric catalysis.
Title: Homogeneous Catalysis
Lecturer(s): Dr R J Cross
Aims: To obtain knowledge of the operations of a wide range of homogeneous catalysis systems in actual applications and to understand how information is derived about the working of such systems.
Objectives: At the end of this course students should be able to show:
1. An ability to recognise and classify the types of organotransition metal reactions occurring in catalytic cycles, and a knowledge of how they operate.
2. A knowledge of hydroformylation, isomerisation, SHOP, metathesis, Ziegler-Natta, Wacker, alkene hydrogenations, and CH bond activation processes.
3. An understanding of how the mechanisms of these processes are elucidated, using spectroscopic, kinetic and isotopic labelling techniques.
4. An ability to interpret mechanistic data and apply these interpretational skills to previously unseen reactions and examples.
5. A knowledge of the merits and demerits of homogeneous and heterogeneous catalysts and an understanding of the basis for catalyst and reaction phase selection for a given process.
6. A knowledge of the methods for introducing asymmetry for enantioselective catalysts.
7. A knowledge of examples of converting molecular catalysts to multi-phase systems by use of surface adsorption, polymer-bound materials or encapsulation.
Outline:
This course covers many types of homogeneous catalysis and includes modern applications ranging from bulk tonnage processes to fine chemical and pharmaceutical manufacture. The reaction types of organo-transition-metal compounds will be related to the operating mechanisms of the catalysts, and emphasis will be placed on the techniques used to obtain and interpret detailed mechanistic information. The techniques described will include modern spectroscopic methods, kinetic studies and the use of isotopic labels. The links between homogeneous and heterogeneous catalysis will be examined, and the advantages and disadvantages of using each type briefly discussed in terms of the factors which govern the choice of suitable catalysts.
Processes examined will include hydroformylation, the SHOP process, Ziegler-Natta polymerisation, Wacker olefin oxidation, olefin hydrogenations (including asymmetric hydrogenations), CH bond activations and the use of anchored transition metal complexes as catalysts.
Title: Simple Fluorides - Reactivity and Catalysis
Lecturer(s): Professor J M Winfield
Aims: To develop the principles that underpin the chemistry of difluorine, fluoride anion, hydrogen fluoride and simple fluorine compounds containing F covalently bound to nitrogen or oxygen.
Objectives:
1. Understand the reasons why F2 is anomolous compared with other X2 halogen molecules and know the implications that these have for fluorine chemistry.
2. Understand the factors that govern the use of F- anion as (i) a strong base and (ii) a good nucleophile.
3. Understand and have some knowledge of, the role of solid oxide supports and catalysts in the use of F- and HF as reagents.
4. Understand the importance of proton donation and hydrogen bonding in the chemistry of HF.
5. Understand the difference between F+ and `electrophilic (positive) fluorine’ in the context of N-F and O-F species.
Outline:
The fluorine anomaly, bond energy and e--e- repulsion considerations. Range of binary fluorides that is known. Ionic fluorides, lattice energy and solvation considerations in nucleophilic fluorination of organics; `naked’ fluoride. Some chemistry derived from F- ion; reactions with Lewis acids, perfluorocarbanions, catalytic behaviour of F- and oxide-supported F- in chlorofluorination of SF4. Structural chemistry of HF, solid, liquid and vapour. Hydrogen bonding and H+ donation in simple HF-base complexes. Catalytic activation of HF, catalytic fluorination of C-Cl bonds under heterogeneous conditions. Complexes between F2 and H2O or amines; electrophilic F and reagents for electrophilic fluorination.
Title: Solid State Chemistry : Materials and Microstructure
Lecturer(s):
Dr D McComb
Aims: To further advance understanding of the inter-relationship between the structure and the properties of materials.
Objectives: At the end of the course students will have a knowledge of the following topics.
1. Review of point defects and ionic conductivity.
2. Properties and applications of fast-ion conductors.
3. Non-stoichiometric compounds: structure and electronic properties.
4. Dislocations and plastic deformation in metals : their influence on mechanical properties.
5. Techniques for investigation of local structural environments.
6. At the end of the course students should be able to describe the properties of a material in terms of the fundamental aspects of solid state chemistry.
Outlines:
Further development of the influence of point defects on the properties of materials. The thermodynamics of formation of point defects as well as the properties/ applications of ionic conductors and solid electrolytes will be studied. The structure-property relationships that result in fast-ion conductors will be considered using appropriate examples. The chemical view regarding the influence of extended defects (dislocations & grain boundaries) on the mechanical properties of materials will be developed. In particular, the relationship between slip planes and dislocations will be developed and plastic deformation behaviour will be considered. For all the objectives examples from recent research literature will be used to illustrate the increasing importance of structure-property relationships in modern solid-state chemistry.
Title: Supramolecular Chemistry
Lecturer(s): Dr. D D MacNicol
Aims: This course aims to provide an introduction to modern supramolecular chemistry, which is concerned with organised entities, consolidated by non-covalent forces, of higher complexity than simple covalently linked molecules. Emphasis will be placed on the design of chiral unimolecular hosts, capable of binding ionic or neutral guests: and the logical design and development of organic lattice inclusion systems will also be highlighted.
Objectives:
1. To understand the origins of modern supramolecular chemistry.
2. To understand the synthetic methods employed to synthesise target receptor molecules.
3. To appreciate how physical methods provide information about the thermodynamics and kinetics of host-guest interactions.
4. To appreciate how special properties, such as drug formulation or enzyme-like catalysis, can be achieved by appropriate host-guest design.
5. To understand the current position on the design of crystalline host lattices: the concepts of symmetry-directed design and the supramolecular synthon.
6. To be familiar with examples of stereochemical control in solid-state reactions; and appreciate the importance of solid-state reactions.
7. To obtain knowledge of existing and potential applications of supramolecular technology.
Course Outline:
Overview of classical discoveries of key organic clathrates and crown ethers; importance of preorganisation — bicyclic and tricyclic effects; electrides; Cram’s chiral crowns, and spherands; Collet’s design of cryptophanes; dynamic NMR studies; cucurbituril catalysis; approaches to crystal design; the supramolecular synthon; solid-state reactions; applications of supramolecular technology; anion activation, optical resolution, supramolecular storage of reagents, inclusion polymerisation; sensor systems.
Title: Enzymes in Organic Chemistry
Lecturer(s):
Dr R A HillAims: To understand the methods involved in determining enzyme mechanisms and to learn how these mechanisms can be applied to using enzymes as reagents and to the design of model enzymes and catalytic antibodies.
Objectives:
1. Understand generally the structures of enzymes.
2. Understand how determination of the stereochemical features of enzyme reactions can be used to determine the mechanisms of these reactions.
3. Describe how the stereochemistry features of alcohol dehydrogenase reactions were established.
4. Describe how the stereochemistry of various enzymic addition/elimination reactions was determined and how this gives an insight into the way enzymes work.
5. Understand how enzymes are such efficient catalysts and how they may be used in organic synthesis.
6. Describe the mechanisms of pyridoxal phosphate enzymes and how they were established.
7. Discuss the use of reductases in synthetic organic chemistry to produce homochiral compounds from achiral compounds and racemic mixtures.
8. Describe the mechanisms and stereochemical features of hydroxylating enzymes.
9. Discuss the use of hydrolytic enzymes in organic reactions.
10. Describe how the concepts of enzymic catalysis have been tested and utilised using enzyme models, catalytic antibodies and biomimetric reactions.
11. Apply the principles of this course in solving problems of related enzyme reactions.
Outline:
The mechanism and stereochemistry of alcohol dehydrogenase and how a knowledge of the stereochemical outcome of alcohol dehydrogenases help the understanding of other enzyme reactions; a series of elimination/addition enzyme reactions will be studied to look at the various methods of examining enzyme mechanisms and to make generalisations about the requirements for efficient enzyme reactions. The enzymes involved are: phenylalanine ammonia lyase, fumarase, aconitase, dehydroquinate dehydrase and aldose-ketose isomerase. Mechanism of pyridoxal phosphate enzymes; the use of enzymes as organic reagents, their advantages and disadvantages; the use of alcohol dehydrogenases including the stereospecific and regiospecific aspects, use in resolution of enantiomers and generation of chiral compounds from achiral substrates; desymmetrisation; hydroxylating enzymes and the mechanism of hydroxylation of unactivated carbons and aromatic compounds including phenylalanine hydroxylase and tyrosine hydroxylase (mention of NIH shift) kinetic isotope effects. Biomimetic chemistry, including intramolecular catalysis, Breslow’s benzophenones, cyclodextrins, cyclophanes, crown ethers and related compounds and catalytic antibodies.
Title: The Chemist’s Approach to Targeting in Medicine
Lecturer: Dr J L Matthews
Aims/Objectives:
The aims and objectives for this course will be issued during lectures. The following is a brief outline of the course.
The course aims to develop an understanding of the uses of organic chemistry in medicine and related fields. The blurring of the traditional boundaries within chemistry is a fact of life in the 1990s. Organic chemistry is at the heart of so many processes and yet its role may not be appreciated by many. This course aims to develop previous understanding of synthetic and other organic chemistry principles (organic synthesis, metal-ion complexation etc.) in such a way as to demonstrate the fundamental role of organic chemistry in today's world using applications taken (in the most part) from medicine. It will demonstrate the essential need for a thorough understanding of basic principles of organic chemistry when considering areas which, at first sight, may not appear to rely on them. Possible topics may include:
1. Magnetic resonance imaging
2. Positron emission tomography
3. Ion sensors
4. Photodynamic therapy.
Title: CNS Pharmacology - Neurotransmitters and Disease
Lecturer(s): Drs F C Boyle and W S Wilson
Aims: To explain the structure and functions of the central nervous system at both the whole animal and cellular levels. To provide an appreciation of the action of endogenous neuroactive agents and of disease processes. To consider the clinical benefits of CNS drugs.
Objectives: Students should appreciate:
1. The structure and functions of the central nervous system.
2. The mechanisms of action of drugs acting upon the central nervous system.
3. The advantages and risks attached to their use in treating disease.
4. The development of new, more selective, potent and safer drugs.
Outline: The overall organisation of the CNS will be described: afferent and efferent systems, general anatomy and function of the CNS and its pathways, leading to a detailed discussion of synaptic transmission, differences from peripheral neuro-transmission, the variety of neurotransmitters and the methods used to identify them. Lectures will cover:
1. An introduction to the structure of the central nervous system
2. Neurotransmitters in the central nervous system
3. Neuroleptic agents
4. GABA and anxiolytic agents
5. Dopaminergic transmission
6. Endogenous opioid transmission
7. Opioid analgesics
8. General anaesthetic agents.