The Sutherland Research Group: Publications
2026
P. K. Majhi, C. M. Fleming and A. Sutherland,
Regioselective Iodination of Arenes Using Iron- or
Silver-Catalyzed Activation of N-Iodosaccharin, J. Org. Chem.,
2026, 91, 2257-2267. DOI:10.1021/acs.joc.5c03183.
.png)
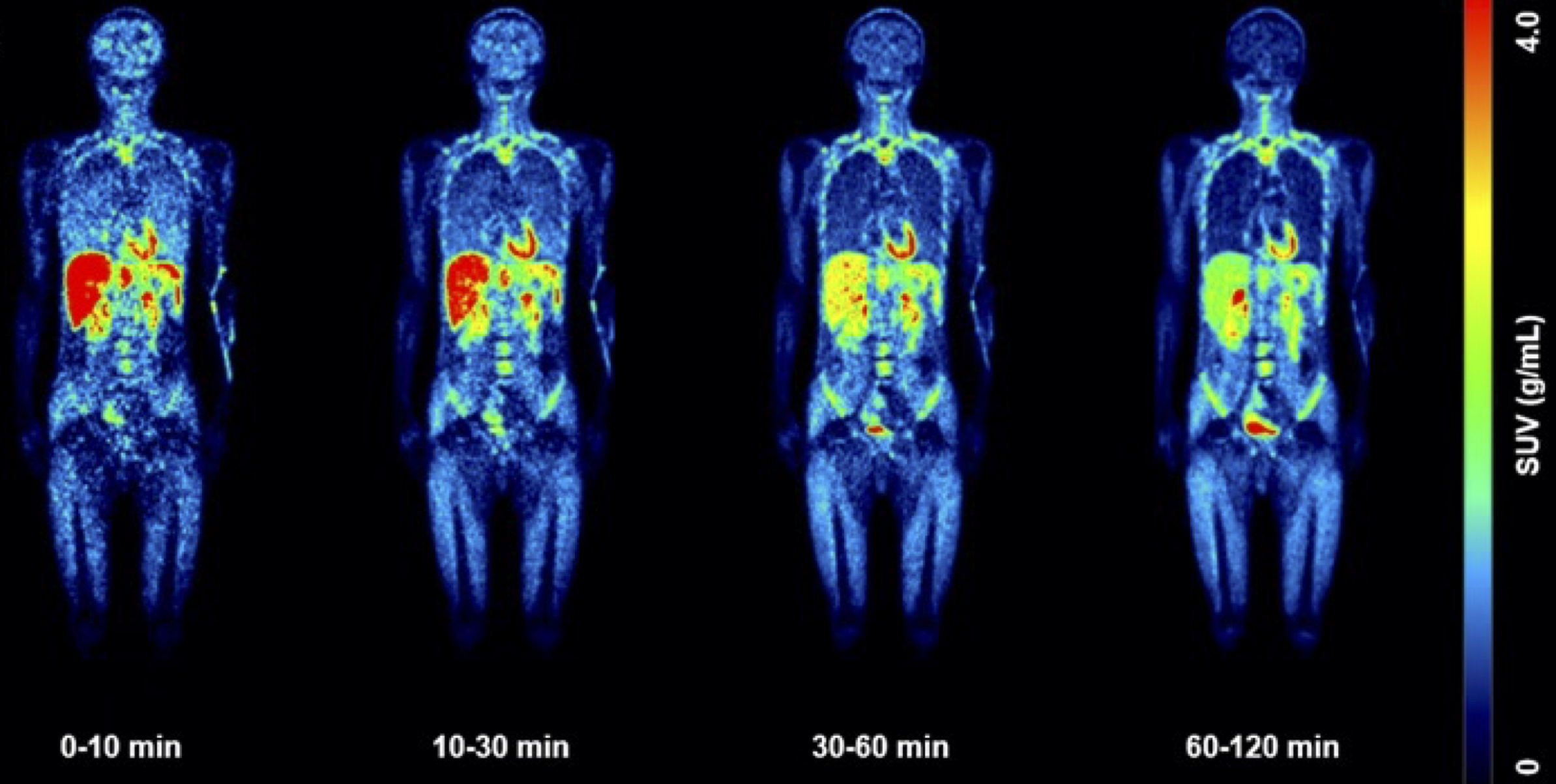
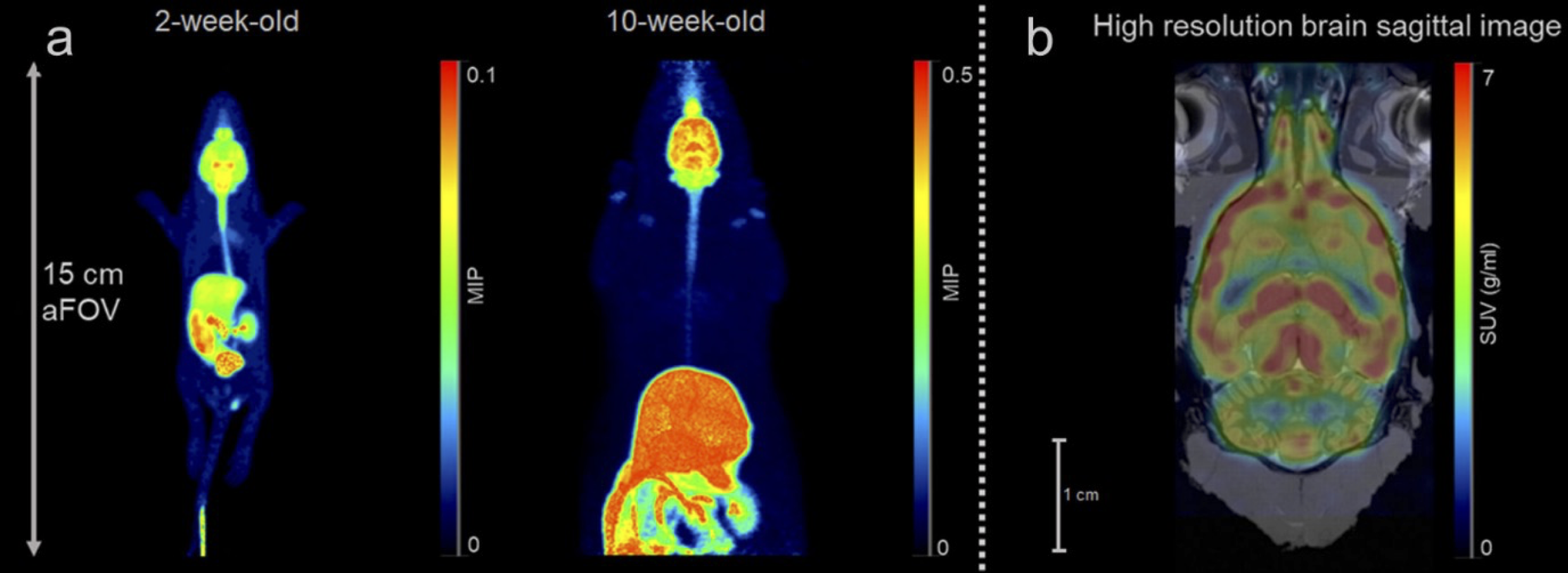
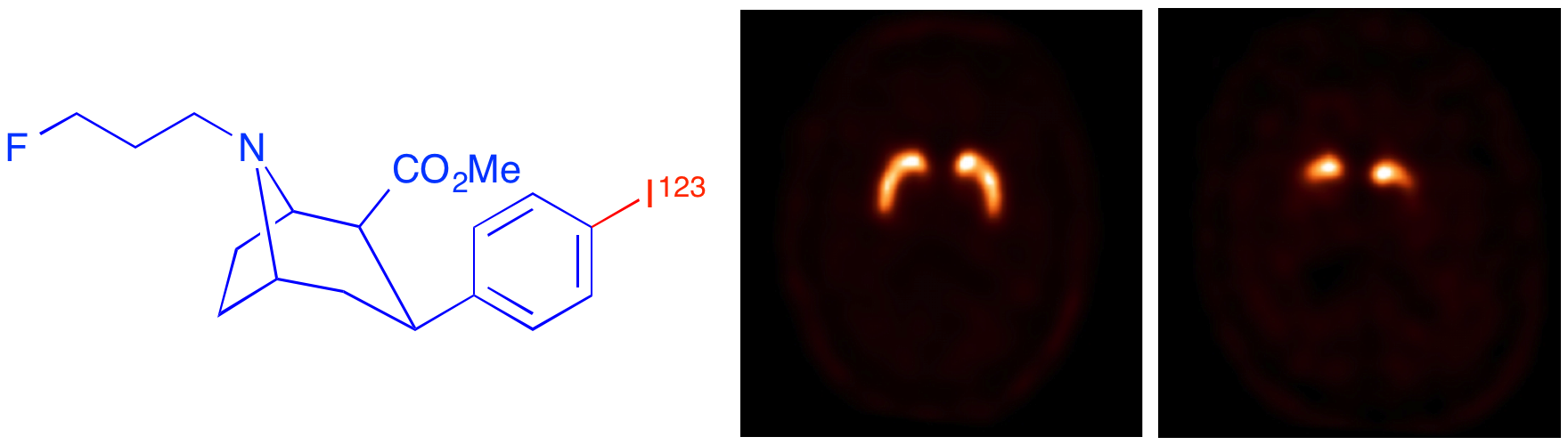
L. Maccioni, A. Knyzeliene, C. J. Alcaide-Corral, V. J. M. Reid, T. E. F. Morgan, M. C. Henry, A. Sutherland, M. Veronese and A. A. S. Tavares, Network-Based Analysis for the Quantification of Brain and Body Immune Axes with Total-Body PET Imaging, J. Nucl. Med., 2026, DOI:10.2967/jnumed.125.271514.
.png)
P. H. Khaing, M. G. MacAskill, J. Xiao, S. Liu,
Z. Gu, X. Sun, T. Xu, N. Koglin, A. W. Stephens, D. E. Newby, Y.
Guan, H. McErlain, A. Sutherland, G. D. Tamagnan, F. Xie and A.
A. S. Tavares, First Human Whole-Body Biodistribution and
Dosimetry Analysis of [18F]LW223, A Novel TSPO PET Radiotracer,
Eur. J. Nucl. Med. Mol. Imag., 2026, DOI:10.1007/s00259-025-07722-0.
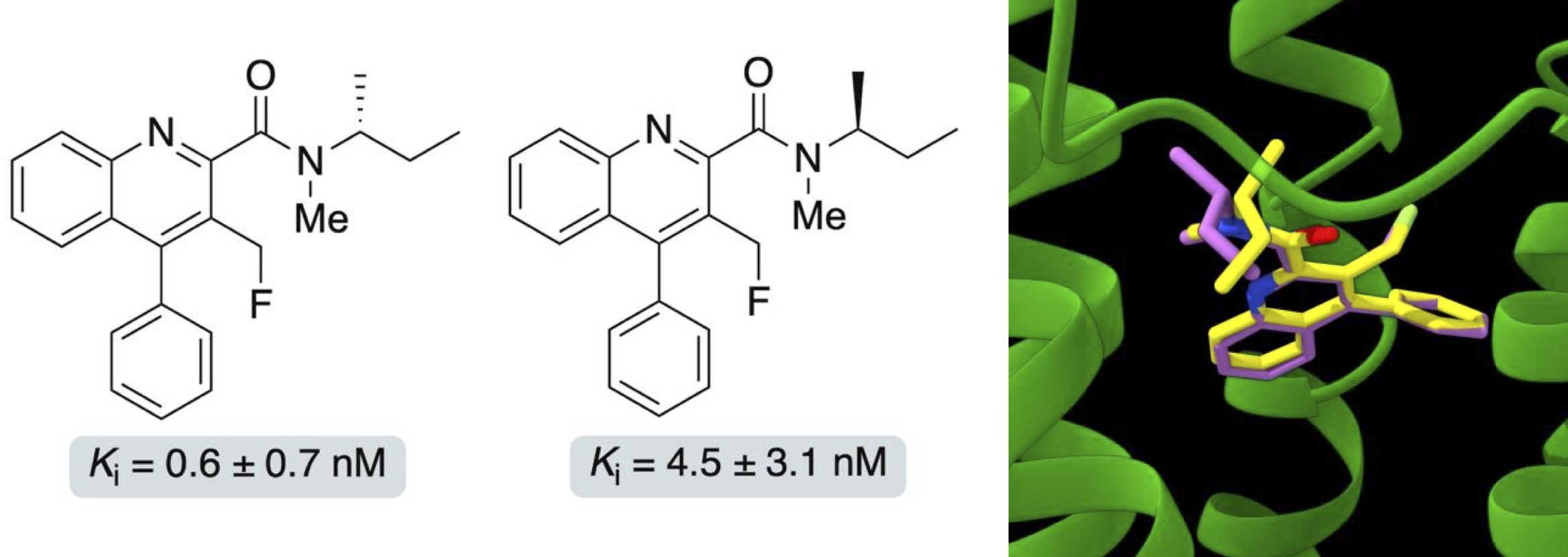
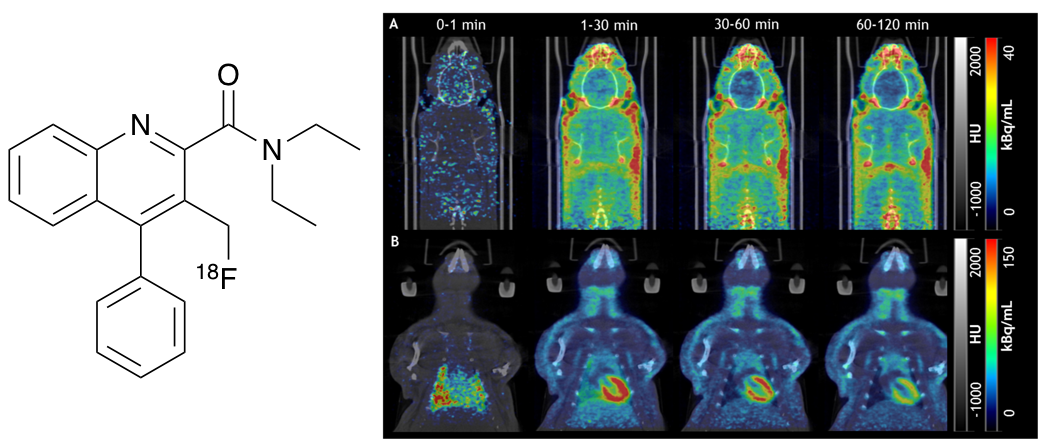
L. J. N. Waddell, M. G. MacAskill, H. McErlain,
T. E. F. Morgan, L. Williams, V. J. Reid, A. Beyger, S. L.
Pimlott, A. A. S. Tavares and A. Sutherland, Synthesis and
Evaluation of Enantiomeric Quinoline-2-Carboxamides: Positron
Emission Tomography Imaging Agents for the Translocator Protein,
RSC Med. Chem., 2026, DOI:10.1039/dmd00930h.
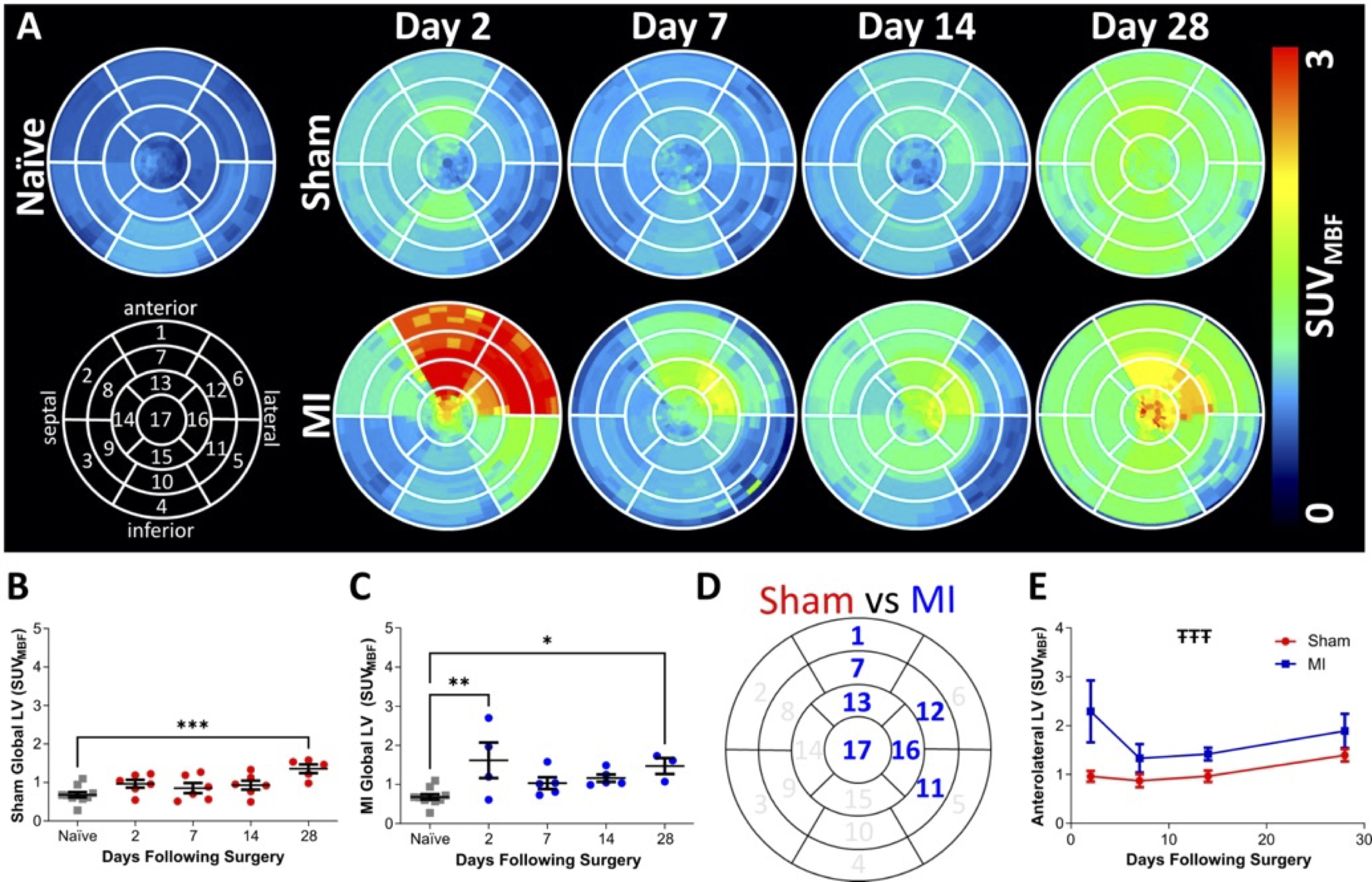
M. G. MacAskill, V. J. M. Reid, C. J.
Alcaide-Corral, T. E. F. Morgan, L. Waddell, A. J. W. Thomson,
T. Fujisawa, N. L. Mills, J. A. Marti, D. Kurian, T. M. Wishart,
A. C. J. De Albuquerque, E. Chui, A. Knyzeliene, V. Balogh, C.
Wimberley, M. R. Dweck, D. E. Newby, C. Lucatelli, S. L.
Pimlott, A. Sutherland and A. A. S. Tavares, Longitudinal
[18F]LW223 PET Imaging of Macrophage-Driven Inflammation
Following Myocardial Infarction in a Rat Model: Implications for
Left Ventricular Remodelling, Eur. J. Nucl. Med. Mol. Imag.,
2026, DOI:10.1007/s00259-025-07691-4.
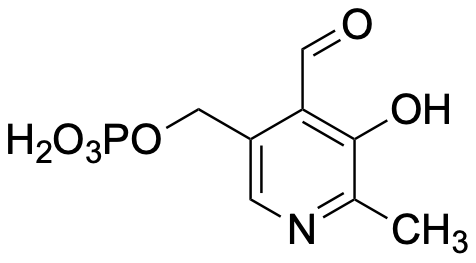
R. S. Dosanjh, S. F. Parker, P. Collier, A.
Pushpanath, A. P. E. York, D. Grainger, S. B. Tailor, T.
Johnson, T. Hyde, L. J. N. Waddell, A. Sutherland and D. Lennon,
A Vibrational Analysis of Pyridoxal 5'-Phosphate Derivatives:
Pyridoxal 5'-Phosphate-Isopropylamine and Pyridoxal
5'-Phosphate-(S)-1-Phenylethylamine. J. Phys. Chem. B.,
2026, 130, 33-45. DOI:10.1021/acs.jpcb.5c04584.
D. Reti, C.-A. Corral, I. Cranston, V. J. M. Reid, K. M. O'Rourke, T. E. F. Morgan, A. Montagne, M. A. Jansen, V. L. Burianova, A. Sutherland, P. Major, K. Nagy, G. Bagamery and A. A. S. Tavares, Performance Evaluation of the NanoScan P123S Total-Body PET, Eur. J. Nucl. Med. Mol. Imag. Phys., 2026, 13, article 2. DOI:10.1186/s40658-025-00817-5.
2025
O. Marshall and A. Sutherland, Expanding the Fluorescence Toolkit: Molecular Design, Synthesis and Biological Application of Unnatural Amino Acids, Chem. Sci., 2025, 16, 16414-16432. DOI:10.1039/d5sc05745k.
.png)
O. Marshall and A. Sutherland, One-Pot Synthesis of Alkynyl-Conjugated Phenylalanine Analogues for Peptide-Based Fluorescent Imaging, Org. Lett., 2025, 27, 8023-8027. DOI:10.1021/acs.orglett.5c02361.
.png)
V. K. Burianova, L. Zeng, A. W. Thom, N. C.
Radcliffe-Kennedy, S. W. Magennis and A. Sutherland,
Environment-Sensitive Fluorecent Tryptophan Analogues via Indole
C-2 Alkenylation Reactions, Chem. Commun., 2025, 61,
11621-11624. DOI:10.1039/d5cc02114f.
.png)
C. Wimberley, C. J. Alcaide-Corral, T. E. F.
Morgan, M. G. Macaskill, B. Andrews, H. McErlain, V. K.
Burianova, A. Sutherland and A. A. S. Tavares, Characterization
of in vivo Binding Kinetics and Non-Displaceable Binding of
[18F]SynVesT-1 in the Rat Brain, Eur. J. Nucl. Med.
Mol. Imag. Res., 2025, 15, 72. DOI:10.1186/s13550-025-01270-2.
.png)
A. Sutherland, M. R. Dweck, D. E. Newby and A. A. S. Tavares,
Total-Body Positron Emission Tomography Imaging to Accelerate
Radiotracer Discovery Pipelines, Pharmacol. Rev., 2025,
77, 100066. DOI:10.1016/j.pharmr.2025.100066.
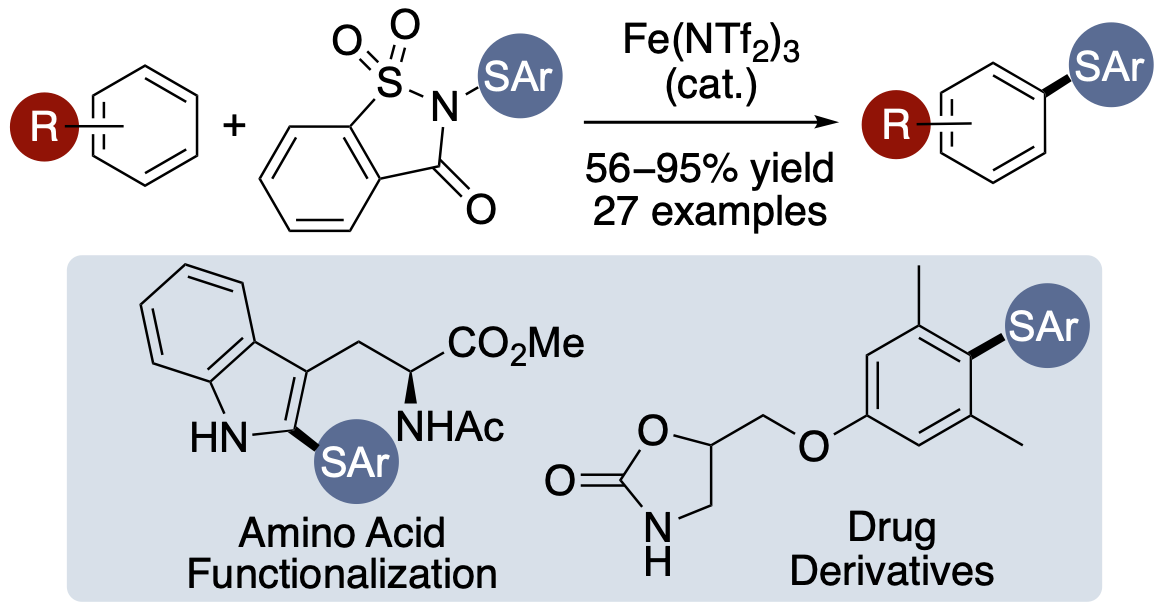
L. J. N. Waddell, O. A. M. Okunade, A. C.
Dodds, M. C. H. Lok, R. N. Khanizeman and A. Sutherland,
Iron-Catalyzed Thioarylation of Arenes Using Saccharin-Derived
Reagents, J. Org. Chem., 2025, 90, 5564-5573. DOI:10.1021/acs.joc.5c00250.
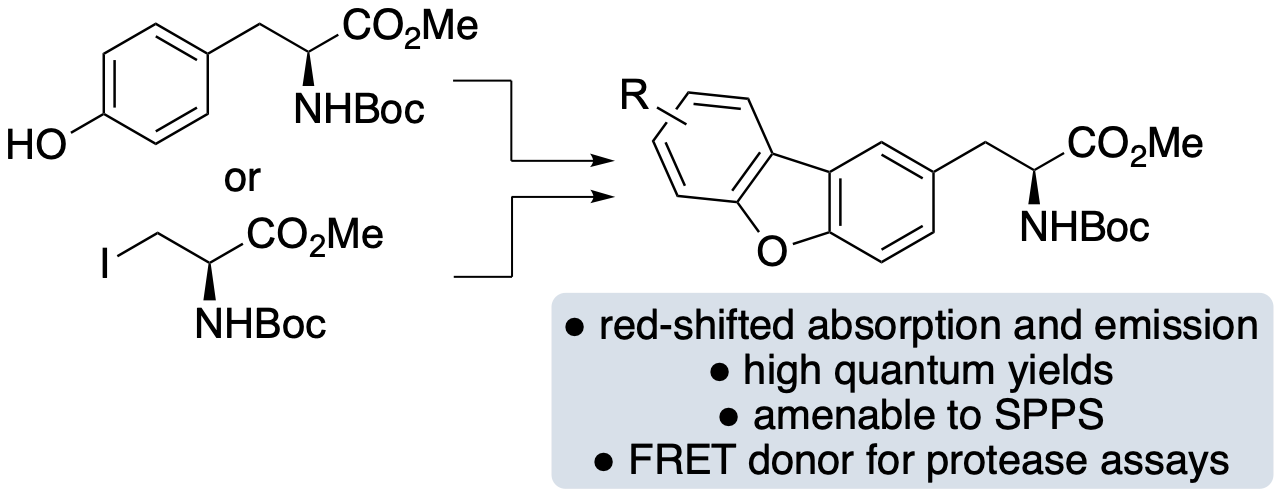
L. Zeng, O. Marshall, R. McGrory, R. Clarke, R. J. Brown, M.
Kadodwala, A. R. Thomson and A. Sutherland, Synthesis of
Fluorescent Dibenzofuran α-Amino
Acids: Conformationally Rigid Analogues of Tyrosine, Org.
Lett., 2025, 27, 2475-2479. DOI:10.1021/acs.orglett.5c00433.
Article was highlighted in SYNFACTS (2025,
21, 649).
O. Marshall, R. McGrory, S. Songsri, A. R. Thomson and A. Sutherland, Expedient Discovery of Fluorogenic Amino Acid-Based Probes via One-Pot Palladium-Catalyzed Arylation of Tyrosine, Chem. Sci., 2025, 16, 3490-3497. DOI:10.1039/d5sc00020c. Selected for the 2025 HOT article collection. Article was highlighted in SYNFACTS (2025, 21, 537).
.png)
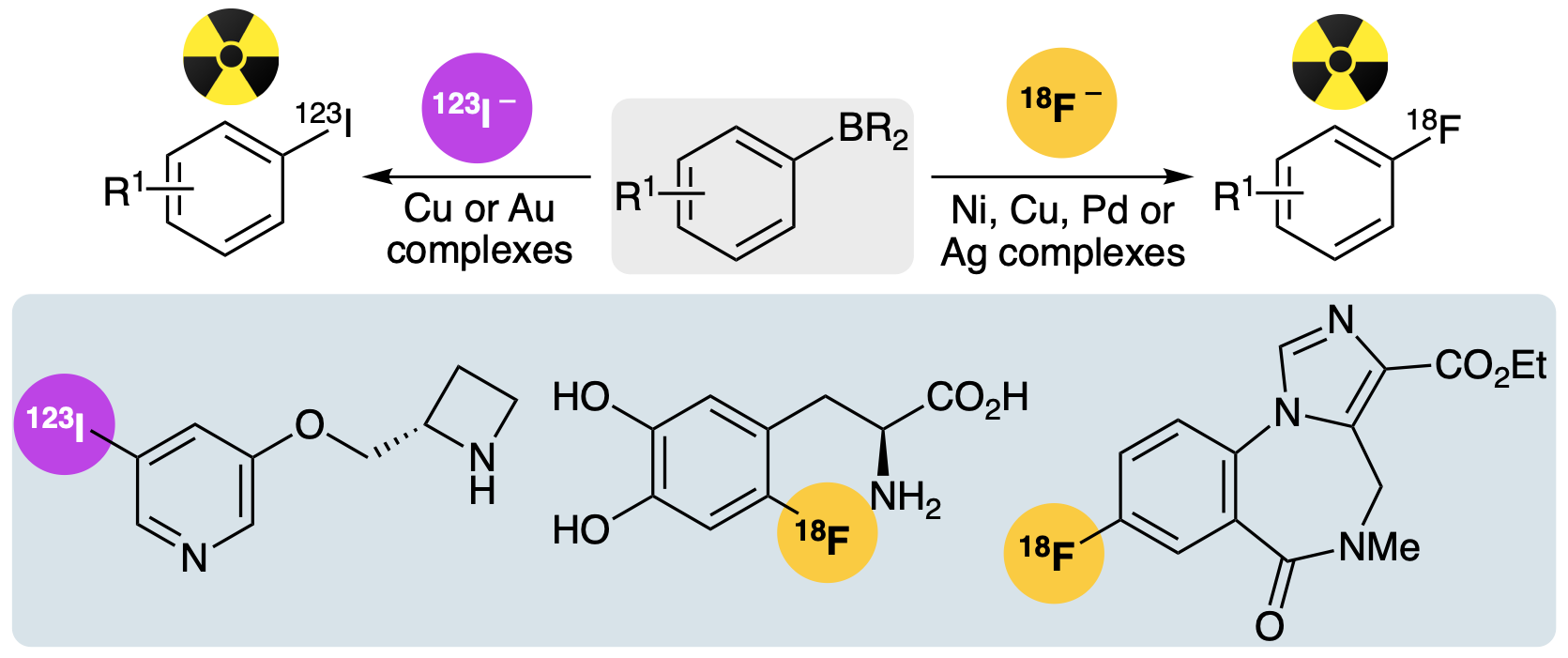
V. K. Burianova, H. McErlain and A. Sutherland, Transition-Metal-Mediated Radiohalogenation using Aryl Boron Reagents, Synthesis, 2025, 57, 1402-1414. DOI:10.1055/s-0043-1775408. Selected by the editorial board for front cover. Article was highlighted in SYNFACTS (2024, 20, 1218).

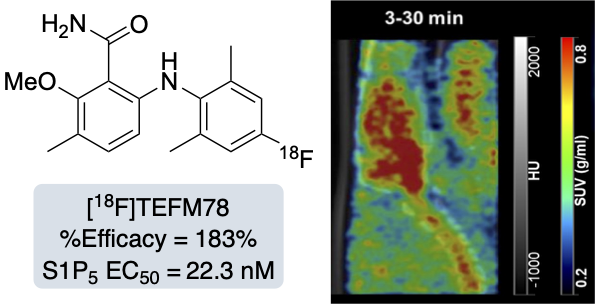
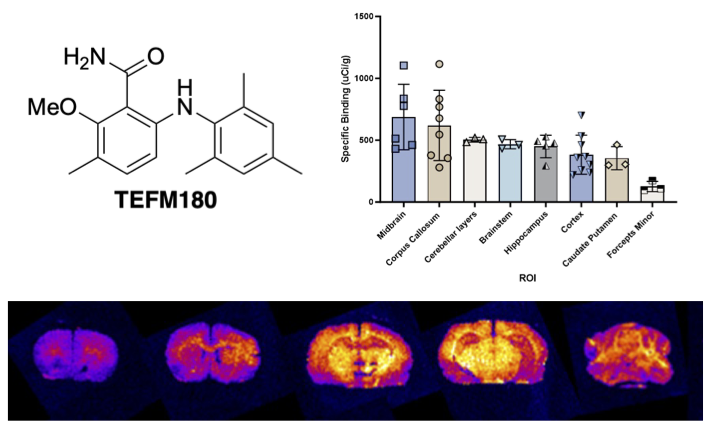
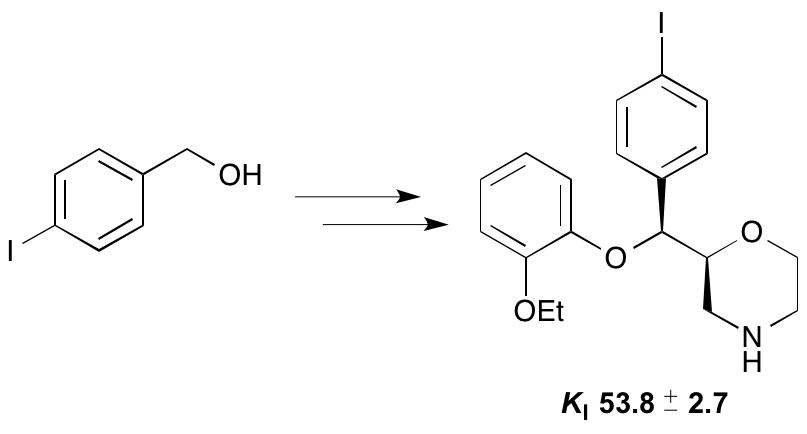
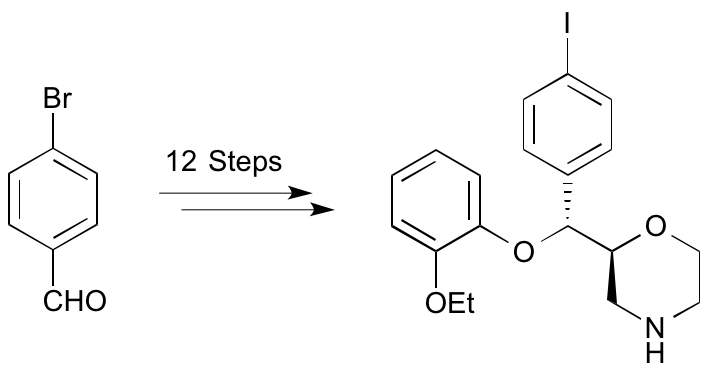
T. E. F. Morgan, E. K. Grant, R. C. Shaw, L. J. N. Waddell, M. C. Henry, H. McErlain, C. J. Alcaide-Corral, S. L. Pimlott, A. A. S. Tavares and A. Sutherland, Synthesis and Evaluation of 6-Arylaminobenzamides as Positron Emission Tomography Imaging Ligands for the Sphingosine-1-phosphate-5 Receptor, RSC Med. Chem., 2025, 16, 1235-1249. DOI:10.1039/d4md00929k.
2024
A. Sutherland, S. Pimlott, A.
Tavares and C. Lucatelli, TSPO Binders. US 12129235 B2,
October 29, 2024.
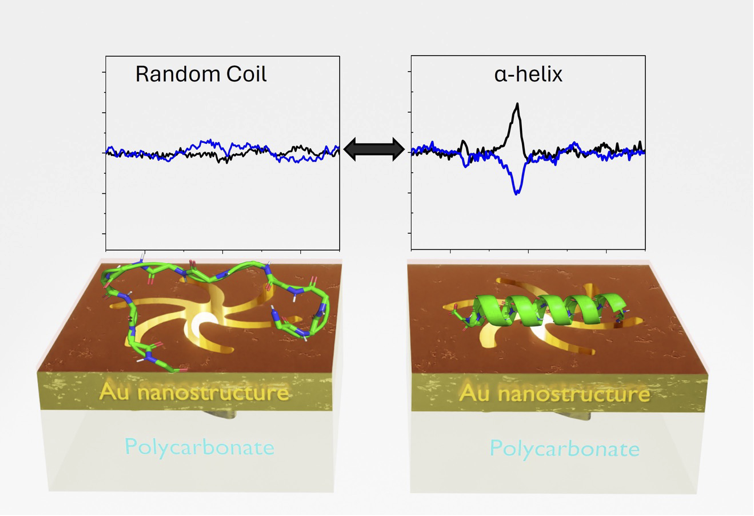
S. K. Chaubey, R. Kumar, P. L. Lalaguna, M.
Kartau, S. Bianco, V. Tabouillot, A. R. Thomson, A. Sutherland,
O. Lyutakov, N. Gadegaard, A. S. Karimullah and M. Kadodwala,
Ultrasensitive Raman Detection of Biomolecular Conformation at
the Attomole Scale using Chiral Nanophotonics, Small,
2024, 20, 2404536. DOI:10.1002/smll.202404536.
S. Songsri, H. McErlain and A. Sutherland,
Stereoselective Synthesis of meso- and L,L-Diaminopimelic
Acids from Enone-Derived α-Amino
Acids, J. Org. Chem., 2024, 89, 10363-10370. DOI:10.1021/acs.joc.4c00916.
.png)
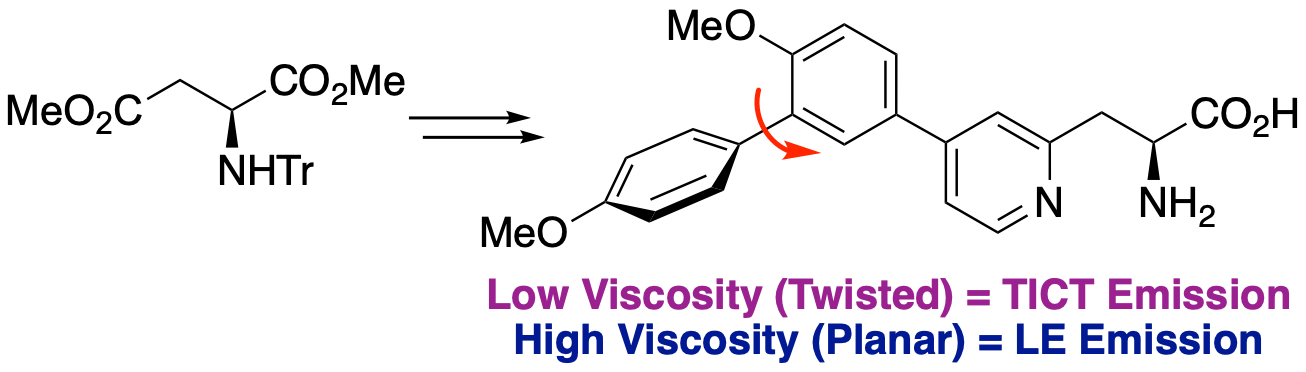
L. M. Riley, O. Marshall, A. H. Harkiss, H. M. Senn and A.
Sutherland, Synthesis of β-Pyridyl α-Amino Acids: Conformationally Sensitive
Charge Transfer-Based Fluorophores, Org. Lett., 2024, 26,
5391-5395. DOI:10.1021/acs.org.lett.4c01951.
H. McErlain, M. J. Andrews, A. J. B. Watson, S.
L. Pimlott and A. Sutherland, Ligand-Enabled Copper-Mediated
Radioiodination of Arenes, Org. Lett., 2024, 26,
1528-1532. DOI:10.1021/acs.orglett.4c00356.
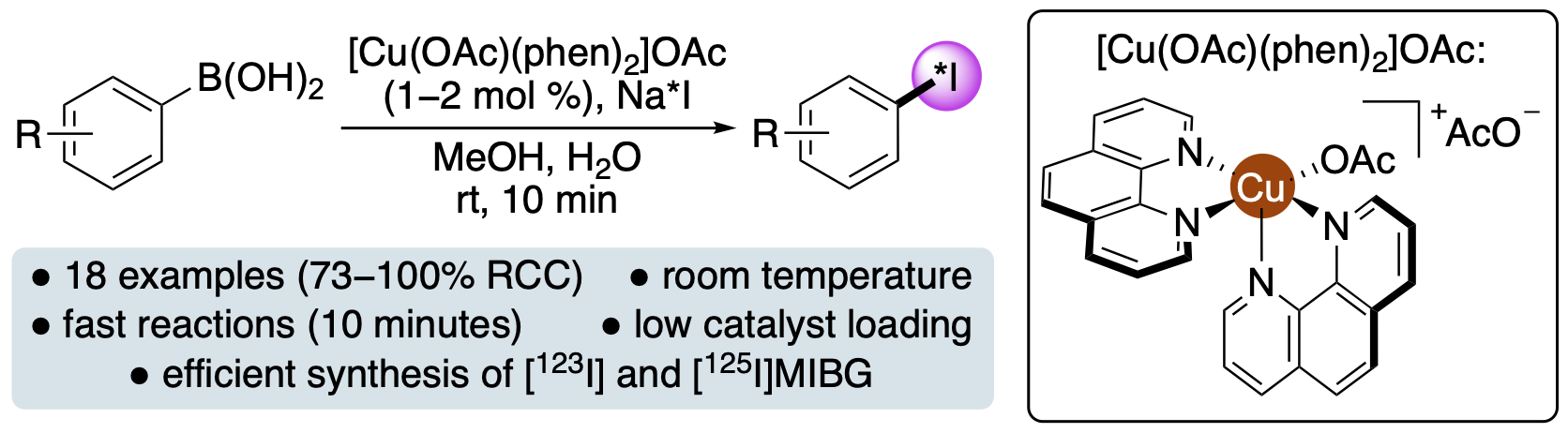
L. J. N. Waddell, C. Wilson and A. Sutherland,
Trifluoromethylthiolation of Arenes Using Lewis Acid and Lewis
Base Dual Catalysis, J. Org. Chem., 2024, 89,
1275-1284. DOI:10.1021/acs.joc.3c02571.
.png)
A. Knyzeliene, M. G. MacAskill, C. J.
Alcaide-Corral, T. E. F. Morgan, M. C. Henry, C. Lucatelli, S.
L. Pimlott, A. Sutherland and A. A. S. Tavares, [18F]LW223 has
Low Non-Displaceable Binding in Murine Brain, Enabling High
Sensitivity TSPO PET Imaging, J. Cereb. Blood Flow Metab.,
2024, 44, 397-406. DOI:10.1177/0271678X231205661.
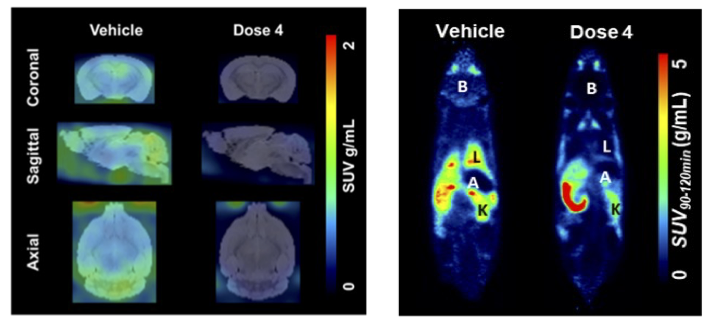
A. Knyzeliene, C. Wimberley, M. G. MacAskill,
C. J. Alcaide-Corral, T. E. F. Morgan, M. C. Henry, C.
Lucatelli, S. L. Pimlott, A. Sutherland and A. A. S. Tavares,
Sexually Dimorphic Murine Brain Uptake of the 18 kDa
Translocator Protein PET Radiotracer [18F]LW223, Brain
Commun., 2024, 6, fcae008. DOI:10.1093/braincomms/fcae008.
.png)
R. A. Hill and A. Sutherland, Hot off the
Press, Nat. Prod. Rep., 2024, 41, 157-161,
520-524, 868-872, 1214-1218, 1466-1470, 1819-1823.
2023
A. C. Dodds, H. G. Sansom, S. W. Magennis and
A. Sutherland, Synthesis of Thiazoloindole α-Amino Acids: Chromophores Amenable to
One- and Two-Photon Induced Fluorescence, Org. Lett.,
2023, 25, 8942-8946. DOI:10.1021/acs.orglett.3c03851.
.png)
S. Songsri, A. H. Harkiss and A. Sutherland, Synthesis and Photophysical Properties of Charge-Transfer-Based Pyrimidine-Derived α-Amino Acids, J. Org. Chem., 2023, 88, 13214-13224. DOI:10.1021/acs.joc.3c01437.
.png)
R. McGrory, R. Clarke, O. Marshall and A. Sutherland, Fluorescent α-Amino Acids via Heck-Matsuda Reactions of Phenylalanine-Derived Arenediazonium Salts, Org. Biomol. Chem., 2023, 21, 6932-6939. DOI:10.1039/d3ob01096a.
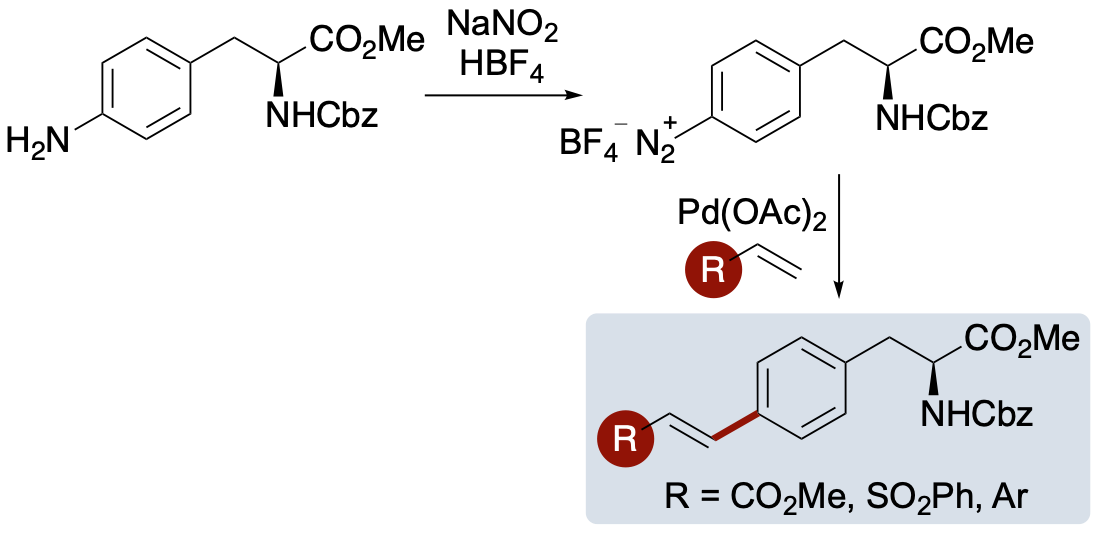
R. McGrory, D. C. Morgan, A. G. Jamieson and A. Sutherland, Rotamer-Controlled Dual Emissive α-Amino Acids, Org. Lett., 2023, 25, 5844-5849. DOI:10.1021/acs.orglett.3c02112.

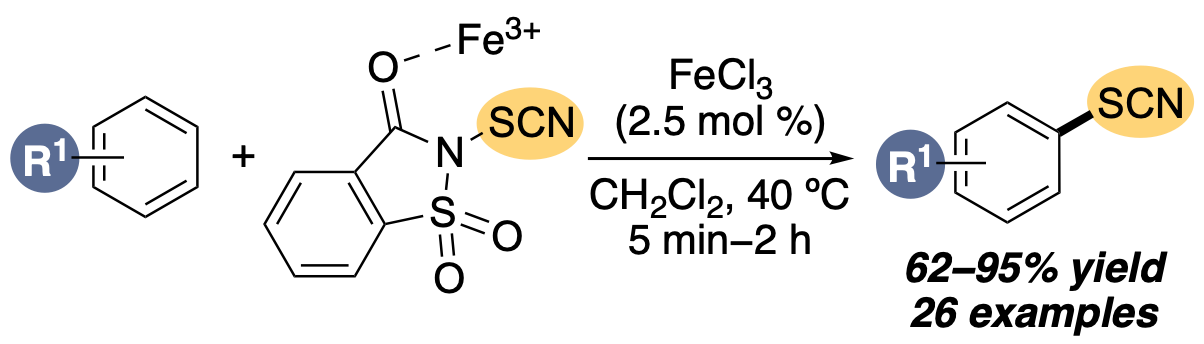
L. J. N. Waddell, M. R. Senkans and A. Sutherland, Regioselective C-H Thiocyanation of Arenes by Iron(III) Chloride, J. Org. Chem., 2023, 88, 7208-7218. DOI:10.1021/acs.joc.3c00454.
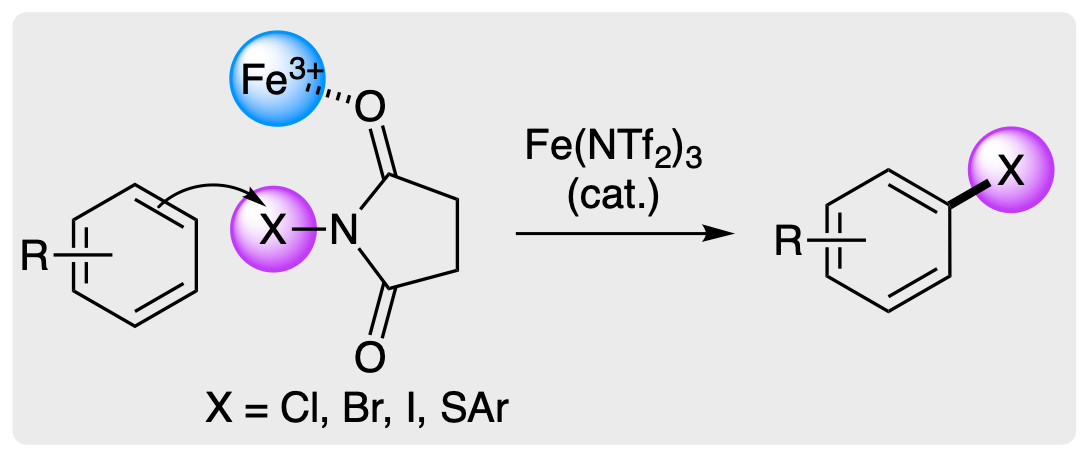
A. C. Dodds, L. J. N. Waddell and A.
Sutherland, Regioselective Functionalization of Arenes Using
Iron Triflimide Catalysis, Synlett, 2023, 34,
1852-1865. DOI:10.1055/s-0042-1751445.
Research Account.
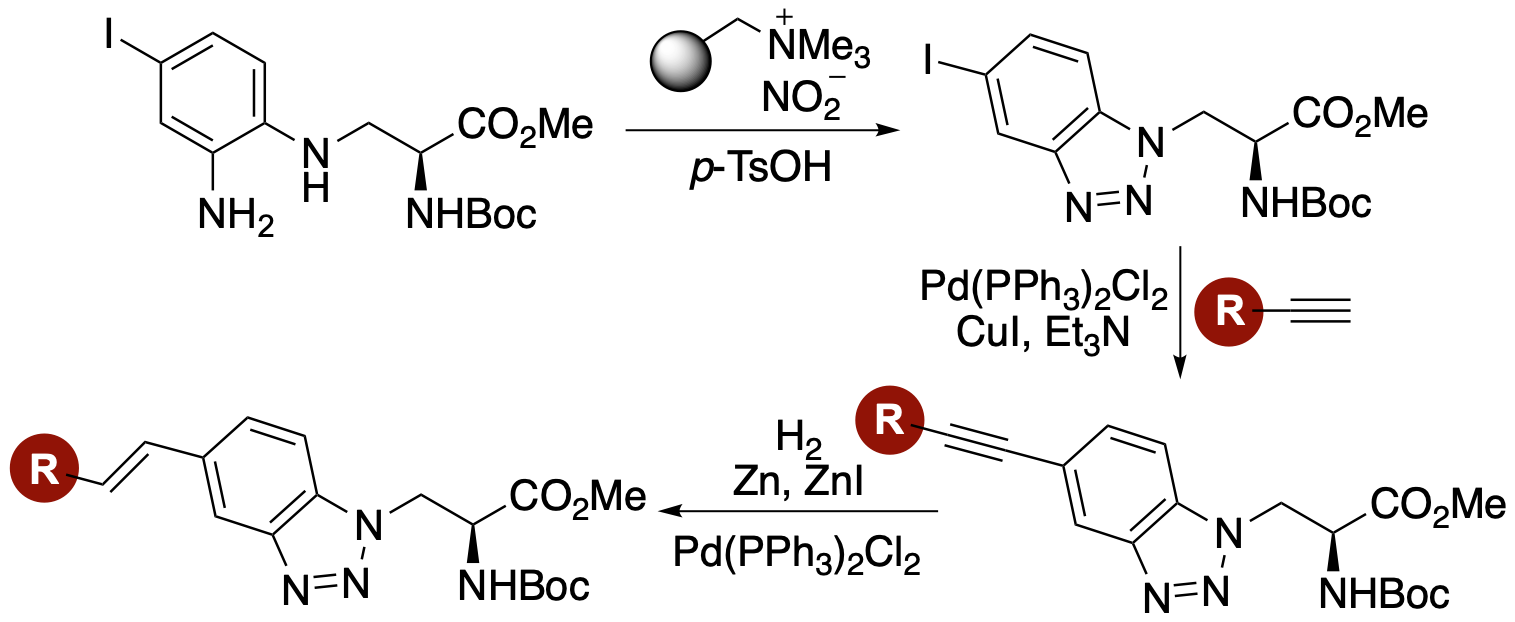
L. M. Riley, T. N Mclay and A. Sutherland,
Synthesis and Fluorescent Properties of Alkynyl- and
Alkenyl-Fused Benzotriazole-Derived α-Amino Acids, J. Org. Chem., 2023, 88,
2453-2463. DOI:10.1021/acs.joc.2c02886.
H. McErlain, E. B. McLean, T. E. F. Morgan, V. K. Burianova, A. A. S. Tavares and A. Sutherland, Organocatalytic Asymmetric Synthesis of SynVesT-1, a Synaptic Density Positron Emission Tomography Imaging Agent, J. Org. Chem., 2022, 87, 14443-14451. DOI:10.1021/acs.joc.2c01895. Article was highlighted in SYNFACTS.

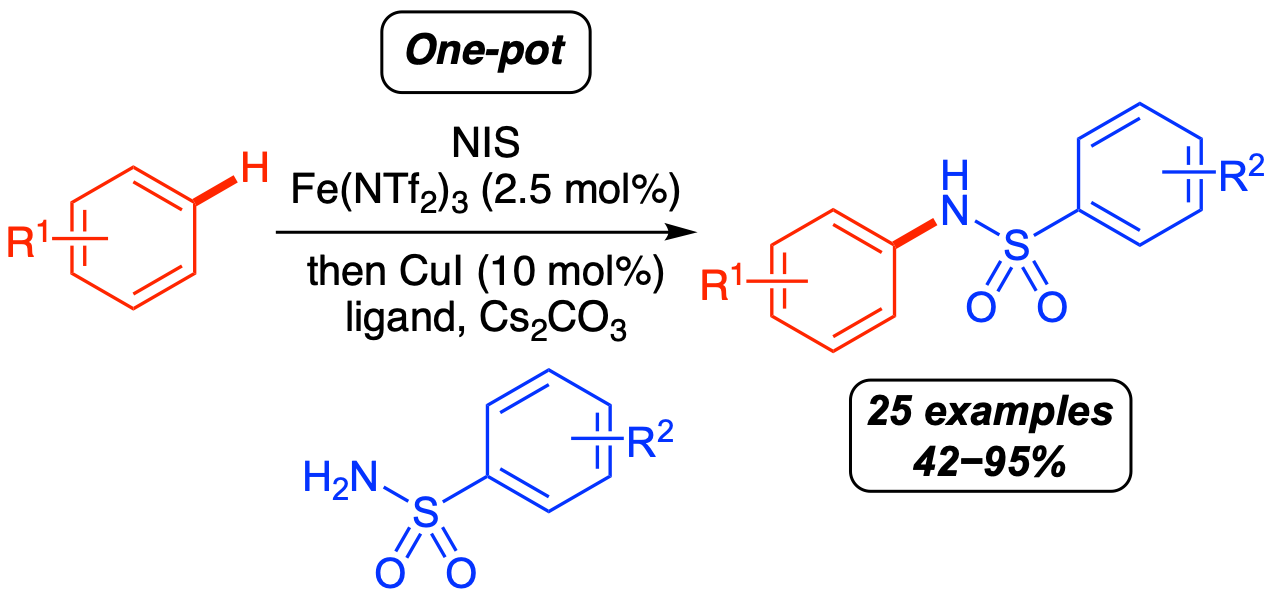
L. J. N. Waddell, M. C. Henry, M. A. B. Mostafa and A. Sutherland, One-Pot Synthesis of Diaryl Sulfonamides using an Iron- and Copper-Catalyzed Aryl C-H Amidation Process, Synthesis, 2022, 54, 4551-4560. DOI:10.1055/a-1884-6988.
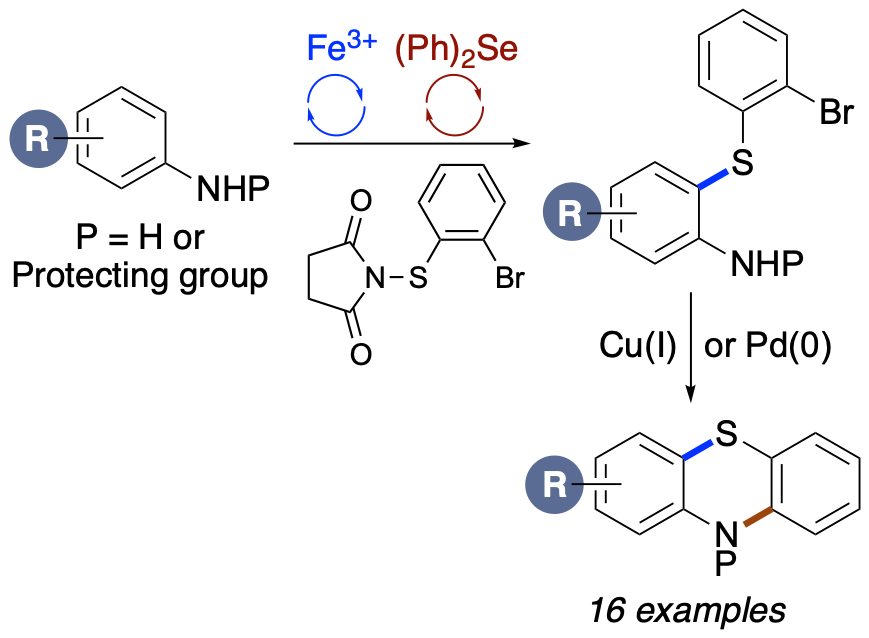
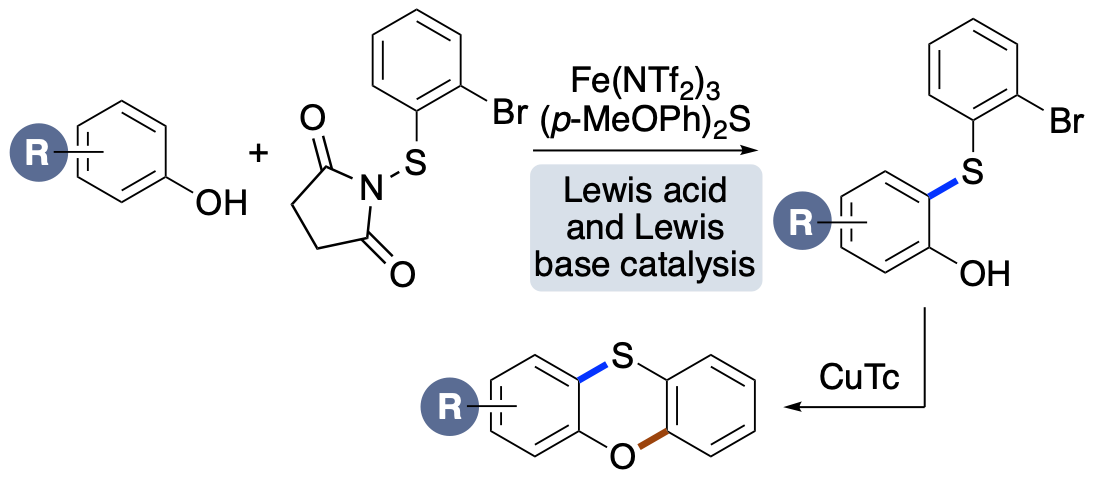
.png)
H. McErlain, L. M. Riley and A. Sutherland, Palladium-Catalyzed C-P Bond Forming Reactions of Aryl Nonaflates Accelerated by Iodide, J. Org. Chem., 2021, 86, 17036-17049. DOI:10.1021/acs.joc.1c02172.
.png)
.png)
R. McGrory, R. J. Faggyas and A. Sutherland, One-Pot Synthesis of N-Substituted Benzannulated Triazoles via Stable Arene Diazonium Salts, Org. Biomol. Chem., 2021, 19, 6127-6140. DOI:10.1039/d1ob00968k. Article was selected for the themed collection: "Synthetic Methodology in OBC".
.png)


M. G. MacAskill, A. Stadulyte, L. Williams, T. E. F. Morgan, N. L. Sloan, C. J. Alcaide-Corral, T. Walton, C. Wimberley, C.-A. McKenzie, N. Spath, W. Mungall, R. BouHaidar, M. R. Dweck, G. A. Gray, D. E. Newby, C. Luctatelli, A. Sutherland, S. L. Pimlott and A. A. S. Tavares, Quantification of Macrophage-Driven Inflammation during Myocardial Infarction with 18F-LW223, a Novel TSPO Radiotracer with Binding Independent of the rs6971 Human Polymorphism, J. Nucl. Med., 2021, 62, 536-544. DOI:10.2967/jnumed.120.243600. JNM Editor's Best Basic Science Article of 2021.

R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2021, 38, 287-291, 677-681, 1053-1057, 1418-1422, 1715-1719, 2139-2144.
2020
E. Dubost, H. McErlain, V. Babin, A. Sutherland and T. Cailly, Recent Advances in Synthetic Methods for Radioiodination, J. Org. Chem., 2020, 85, 8300-8310. DOI:10.1021/acs.joc.0c00644. ACS Editors' Choice.


M. C. Henry and A. Sutherland, Synthesis of Benzo[b]furans by Intramolecular C-O Bond Formation Using Iron and Copper Catalysis, Org. Lett., 2020, 22, 2766-2770. DOI:10.1021/acs.orglett.0c00754.
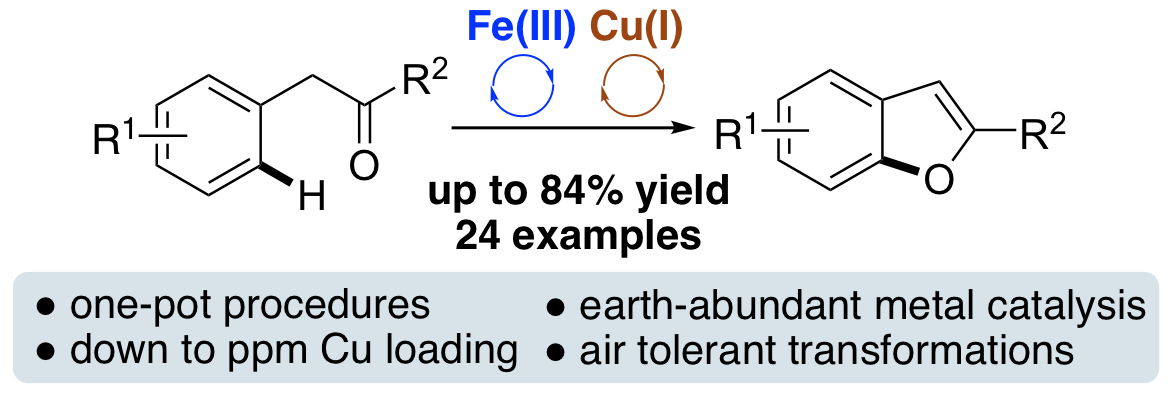
D. E. Sood, S. Champion, D. Dawson, S. Chabbra, B. Bode, A. Sutherland and A. J. B. Watson, Deoxyfluorination using CuF2 Enabled by a Lewis Base Activating Group Strategy, Angew. Chem. Int. Ed., 2020, 59, 8460-8463. DOI:10.1002/anie.202001015.

M. C Henry, V. M. Abbinante and A. Sutherland, Iron-Catalyzed Regioselective Synthesis of 2-Arylbenzoxazoles and 2-Arylbenzothiazoles via Alternative Reaction Pathways, Eur. J. Org. Chem., 2020, 2819-2826. DOI:10.1002/ejoc.202000014.

J. D. Bell, A. H. Harkiss, D. Nobis, E. Malcolm, A. Knuhtsen, C. R. Wellaway, A. G. Jamieson, S. W. Magennis and A. Sutherland, Conformationally Rigid Pyrazoloquinazoline α-Amino Acids: One- and Two-Photon Induced Fluorescence, Chem. Commun., 2020, 56, 1887-1890. DOI:10.1039/C9CC09064A.
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2020, 37, 145-149, 472-476, 747-751, 1038-1042, 1294-1299, 1556-1560.
2019
A. Sutherland, S. Pimlott, A. Tavares and C. Lucatelli, TSPO Binders. W.O. Patent WO2019243616 A1, December 26, 2019.
J. D. Bell, T. E. F. Morgan, N. Buijs, A. H. Harkiss, C. R. Wellaway and A. Sutherland, Synthesis and Photophysical Properties of Benzotriazole-Derived Unnatural α-Amino Acids, J. Org. Chem., 2019, 84, 10436-10448. DOI:10.1021/acs.joc.9b01685.
A. Sutherland, Radiohalogenation of Organic Compounds: Practical Considerations and Challenges for Molecular Imaging, Synthesis, 2019, 51, 4368-4373. DOI:10.1055/s-0037-1611885. Invited submission for a special topic: Halogenation methods (with a view towards radioimaging applications). Article Featured on the Front Cover.
M. C. Henry, R. McGrory, R. J. Faggyas, M. A. B. Mostafa and A. Sutherland, One-Pot ortho-Amination of Aryl C-H Bonds Using Consecutive Iron and Copper Catalysis, Org. Biomol. Chem., 2019, 17, 4629-4639. DOI:10.1039/C9OB00712A. Article was selected for the themed collection: "Synthetic Methodology in OBC".
R. J. Faggyas, N. L. Sloan, N. Buijs and A. Sutherland, Synthesis of Structurally Diverse Benzotriazoles via Rapid Diazotization and Intramolecular Cyclization of 1,2-Aryldiamines, Eur. J. Org. Chem., 2019, 5344-5353. DOI:10.1002/ejoc.201900463. Invited contribution to a special issue on "Heterocycles". Article was also featured on ChemistryViews.

M. G. MacAskill, T. Walton, L. Williams, T. E. F. Morgan, C. J. Alcaide-Corral, M. R. Dweck, G. A. Gray, D. E. Newby, C. Lucatelli, A. Sutherland, S. L. Pimlott, A. A. S. Tavares, Kinetic Modelling and Quantification Bias in Small Animal PET Studies with [18F]AB5186, a Novel 18 kDa Translocator Protein Radiotracer, PLoS ONE, 2019, 14, e0217515. DOI:10.1371/journal.pone.0217515.
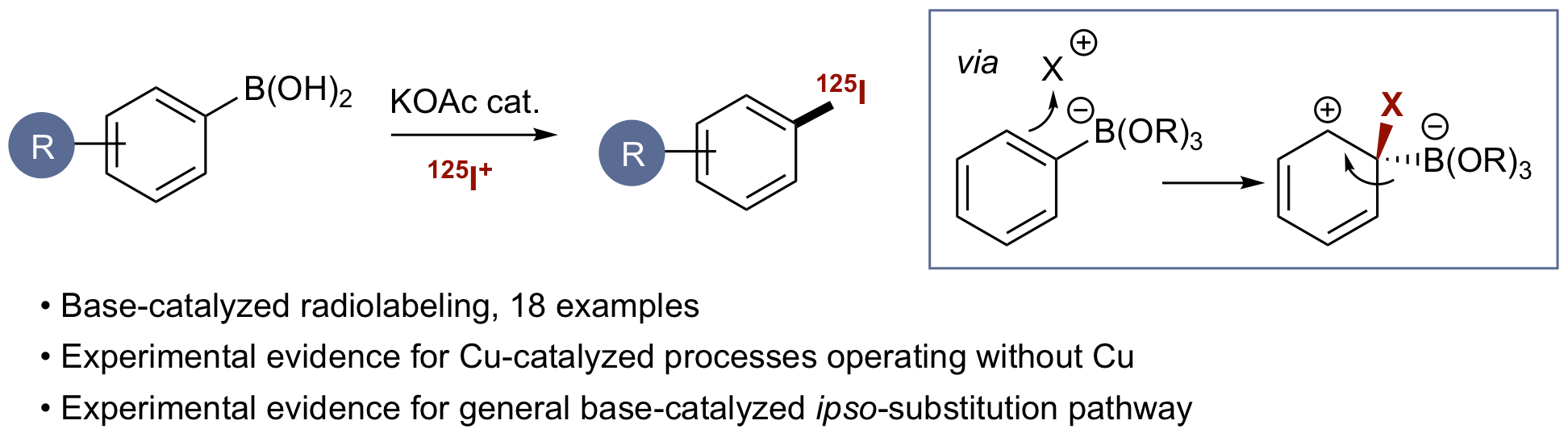
J. J. Molloy, K. M. O'Rourke, C. P. Frias, N. L. Sloan, M. J. West, S. L. Pimlott, A. Sutherland and A. J. B. Watson, Mechanism of Cu-Catalyzed Aryl Boronic Acid Halodeboronation Using Electrophilic Halogen: Development of a Base-Catalyzed Iododeboronation for Radiolabeling Applications, Org. Lett., 2019, 21, 2488-2492. DOI:10.1021/acs.orglett.9b00942. Article was featured on the Organic Chemistry Portal.
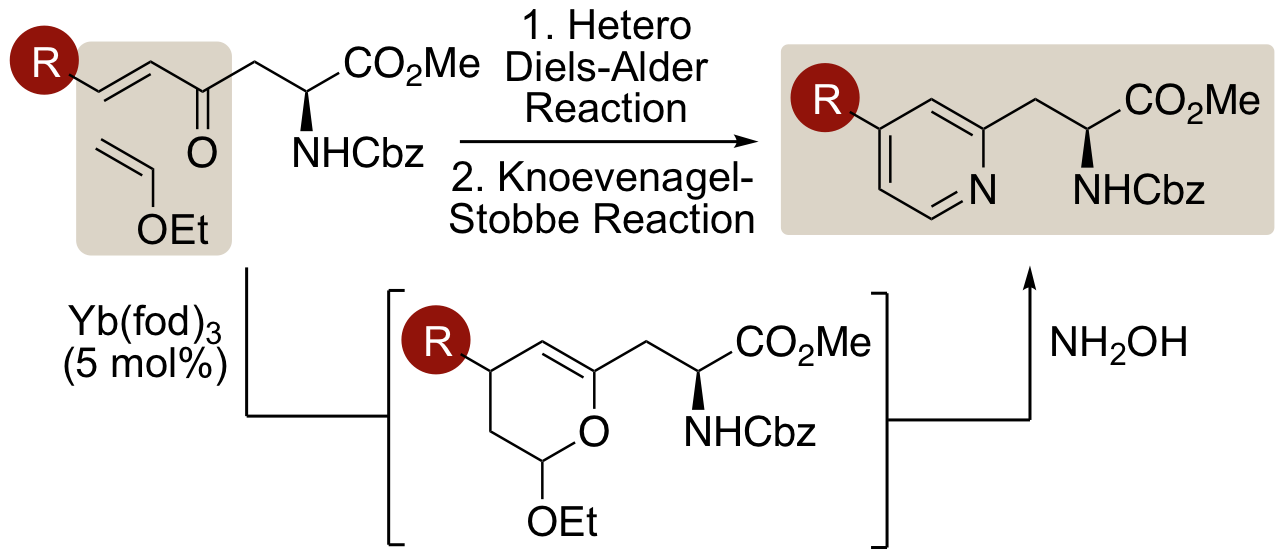
A. H. Harkiss, J. D. Bell, A. Knuhtsen, A. G. Jamieson and A. Sutherland, Synthesis and Fluorescent Properties of β-Pyridyl α-Amino Acids, J. Org. Chem., 2019, 84, 2879-2890. DOI:10.1021/acs.joc.9b00036. Article was featured on the Organic Chemistry Portal.
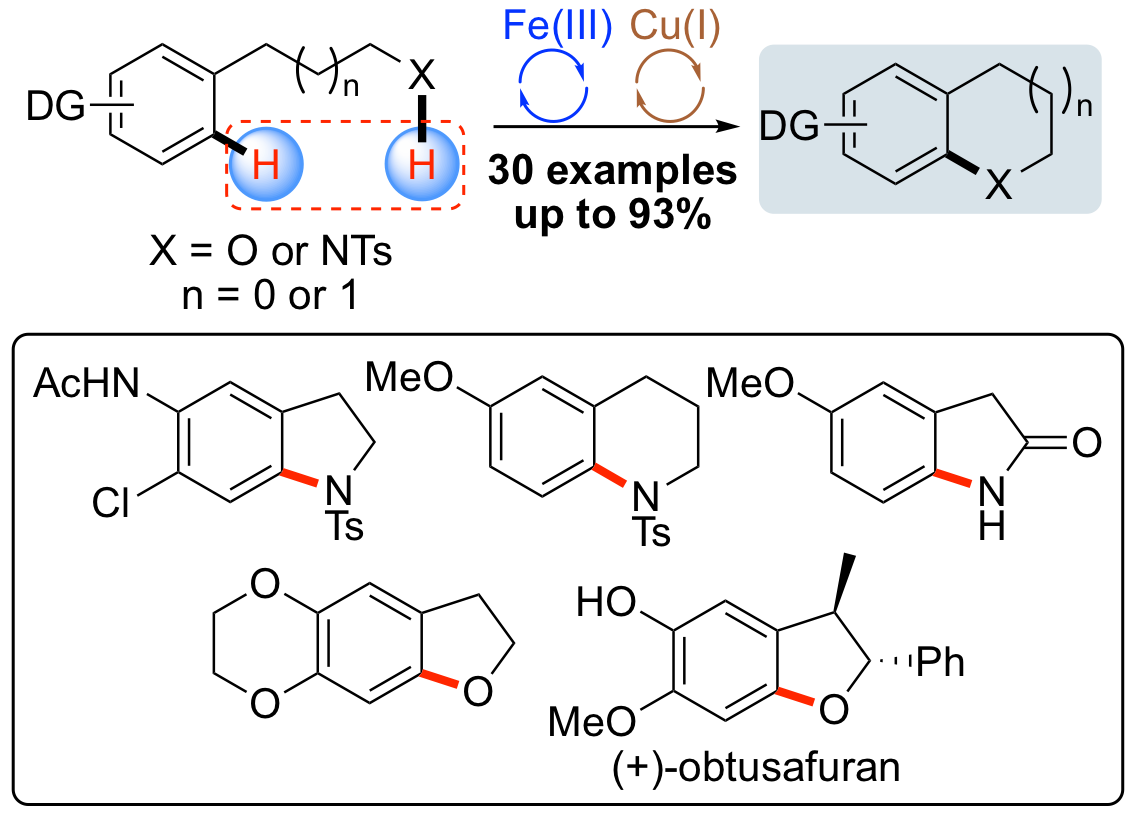
M. C. Henry, H. M. Senn and A. Sutherland, Synthesis of Functionalized Indolines and Dihydrobenzofurans by Iron and Copper Catalyzed Aryl C-N and C-O Bond Formation, J. Org. Chem., 2019, 84, 346-364. DOI:10.1021/acs.joc.8b02888.
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2019, 36, 258-262, 556-560, 850-854, 1039-1043, 1378-1382, 1614-1618.
2018
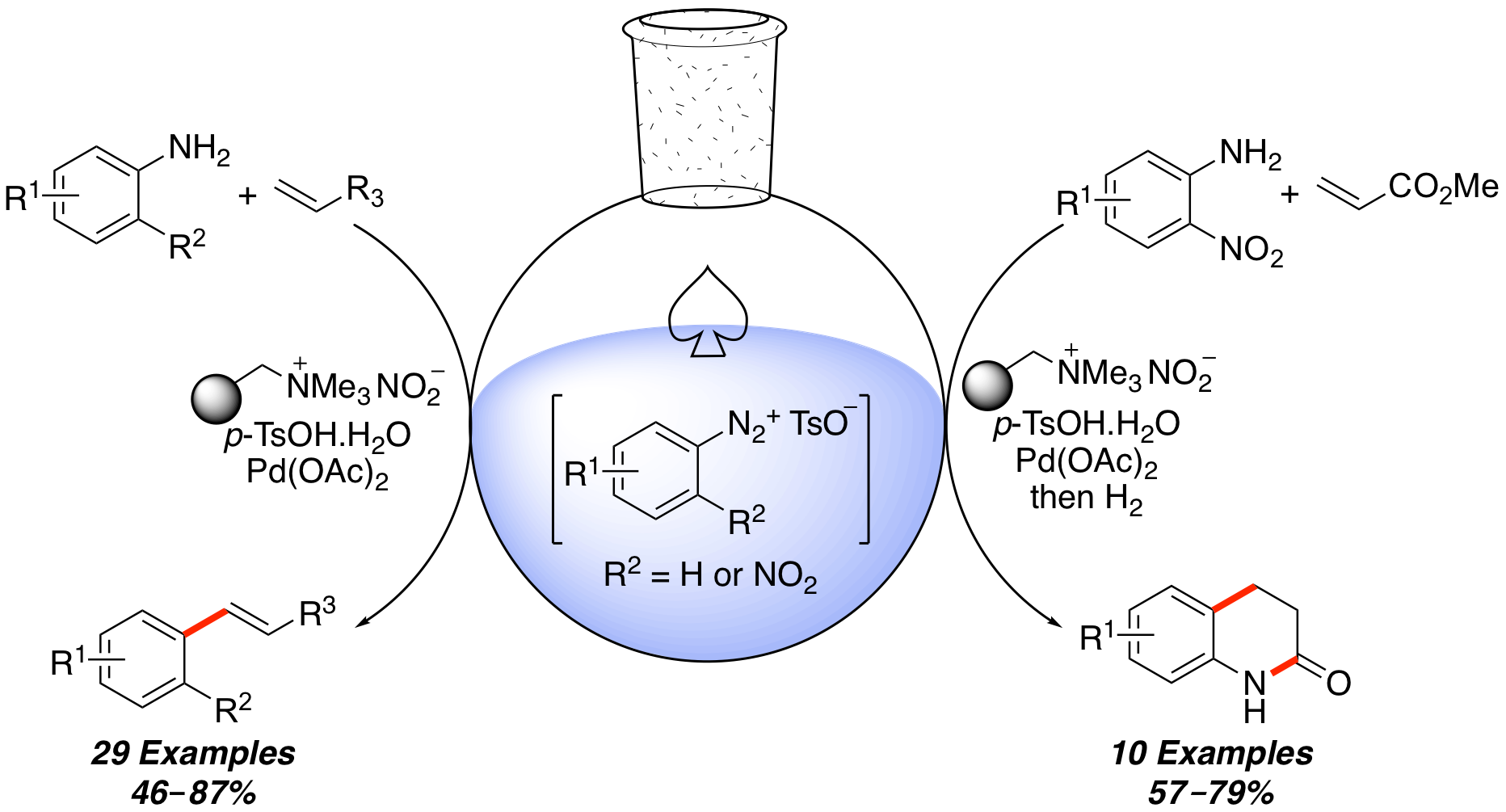
R. J. Faggyas, M. Grace, L. Williams and A. Sutherland, Multibond Forming Tandem Reactions of Anilines via Stable Aryl Diazonium Salts: One-Pot Synthesis of 3,4-Dihydroquinolin-2-ones, J. Org. Chem., 2018, 83, 12595-12608. DOI:10.1021/acs.joc.8b01910.
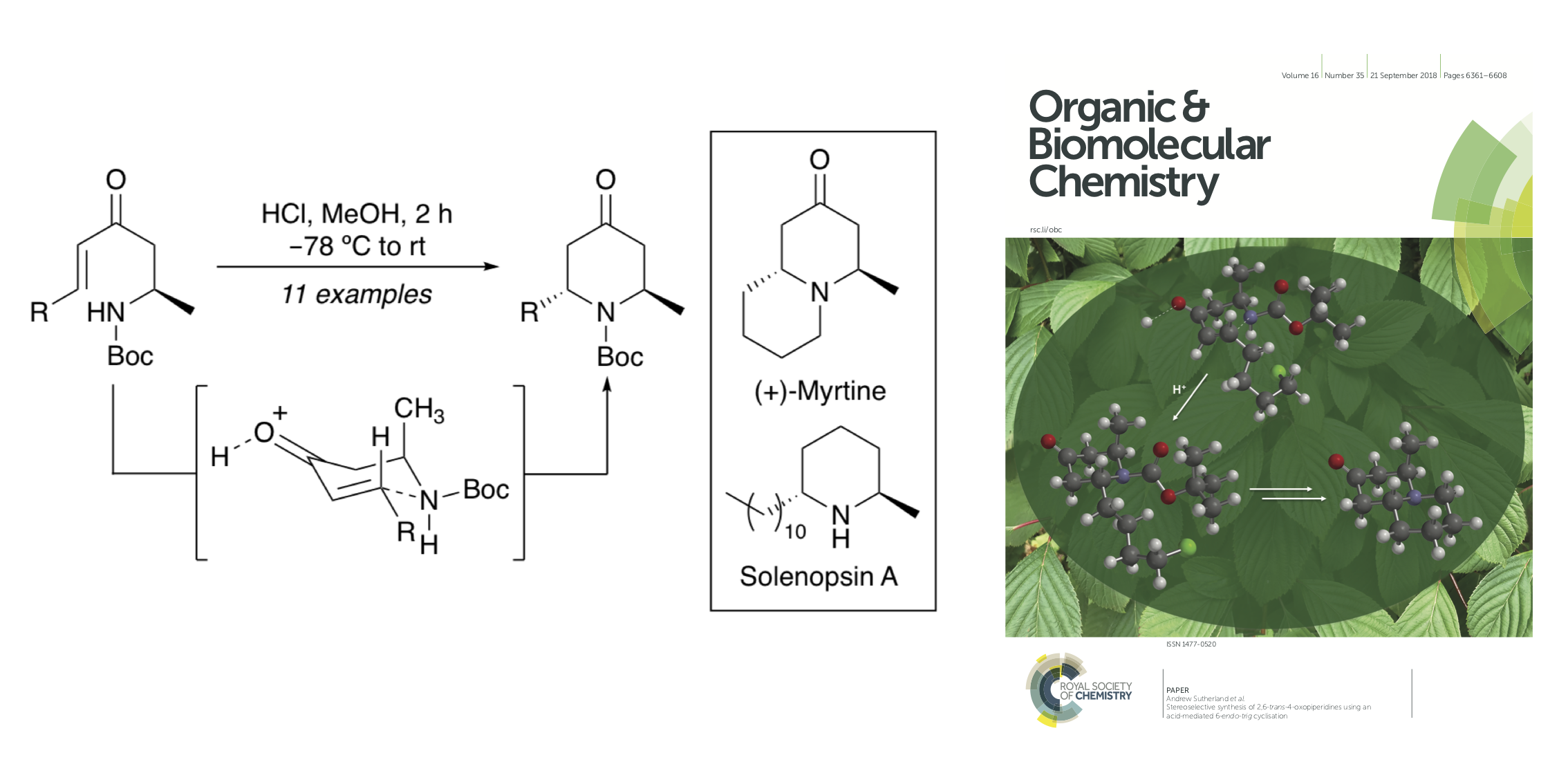
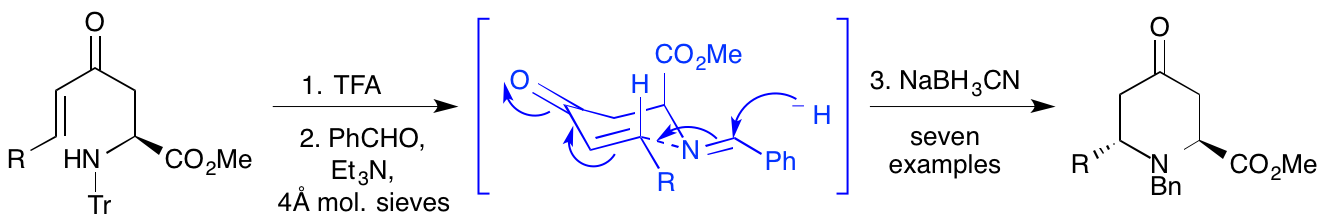
J. D. Bell, A. H. Harkiss, C. R. Wellaway and A. Sutherland, Stereoeselective Synthesis of 2,6-trans-4-Oxopiperidines using an Acid-Mediated 6-endo-trig Cyclisation, Org. Biomol. Chem., 2018, 16, 6410-6422. DOI:10.1039/C8OB01363B. Front Cover of Journal. Article was selected for the themed collections: "Synthetic Methodology in OBC" and "Organic and Biomolecular Chemistry HOT article collection."
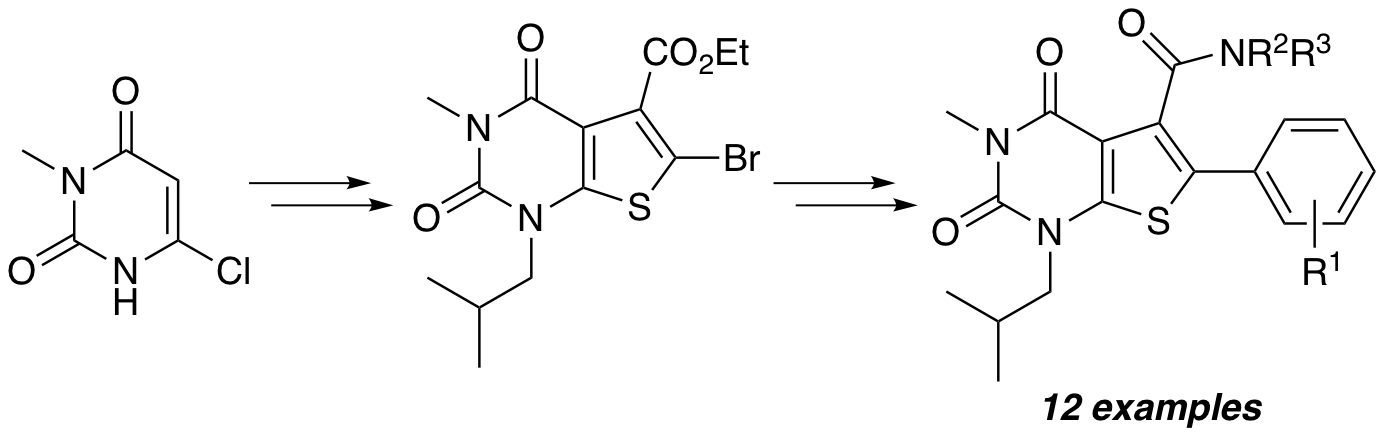
K. M. O’Rourke, E. S. Johnstone, H. M. Becker, S. L. Pimlott and A. Sutherland, Exploring the Functionalisation of the Thieno[2,3-d]pyrimidinedione Core: Late Stage Access to Highly Substituted 5-Carboxamide-6-Aryl Scaffolds, Tetrahedron, 2018, 74, 4086-4094. DOI:10.1016/j.tet.2018.06.019.
F. Zmuda, A. Blair, M. C. Liuzzi, G. Malviya, A. J. Chalmers, D. Lewis, A. Sutherland and S. L. Pimlott, An 18F-labeled Poly(ADP-ribose) Polymerase Positron Emission Tomography Imaging Agent, J. Med. Chem. 2018, 61, 4103-4114. DOI:10.1021/acs.jmedchem.8b00138.
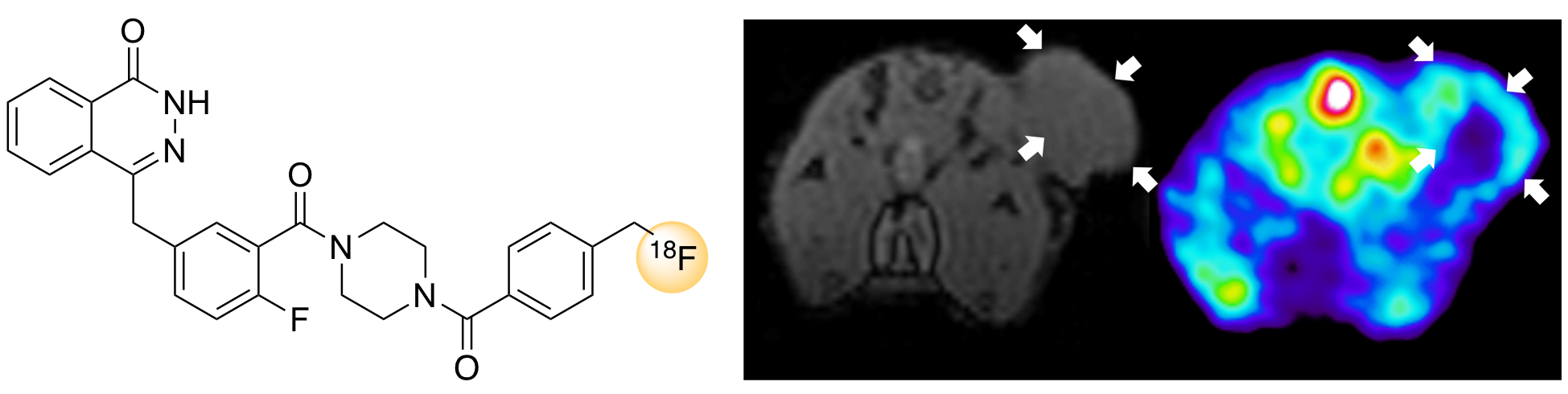
E. K. Gibson, J. Callison, J. M. Winfield, A. Sutherland, R. H. Carr, A. Eaglesham, S. F. Parker and D. Lennon, Spectroscopic Characterisation of Model Compounds, Reactants and Byproducts Connected with an Isocyanate Production Chain, Ind. Eng. Chem. Res., 2018, 57, 7355-7362. DOI:10.1021/acs.icer.8b00853.
S. Webster, K. M. O'Rourke, C. Fletcher, S. L. Pimlott, A. Sutherland and A.-L. Lee, Rapid Iododeboronation with and without Gold Catalysis: Application to Radiolabelling of Arenes, Chem. Eur. J., 2018, 24, 937-943. DOI:10.1002/chem.201704534. Highlighted in Synfacts (2018, 14, 365).
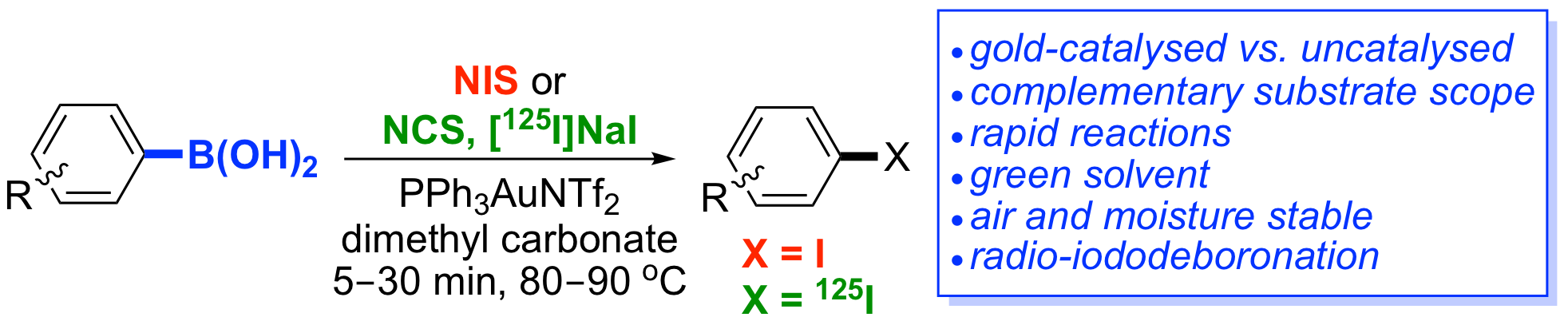
A. H. Harkiss and A. Sutherland, Access to 2,6-Disubstituted 4-Oxopiperidines Using a 6-endo-trig Cyclization: Stereoselective Synthesis of Spruce Alkaloid and (+)-241D, J. Org. Chem., 2018, 83, 535-542. DOI:10.1021/acs.joc.7b02799.
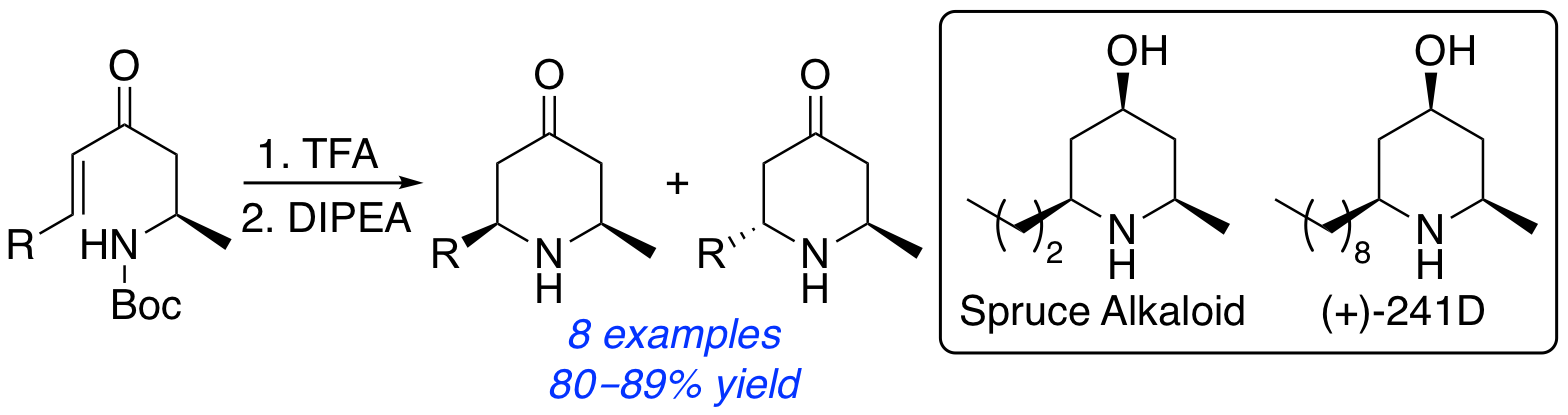
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2018, 35, 132-136, 298-302, 496-500, 702-706, 1024-1028, 1236-1240.
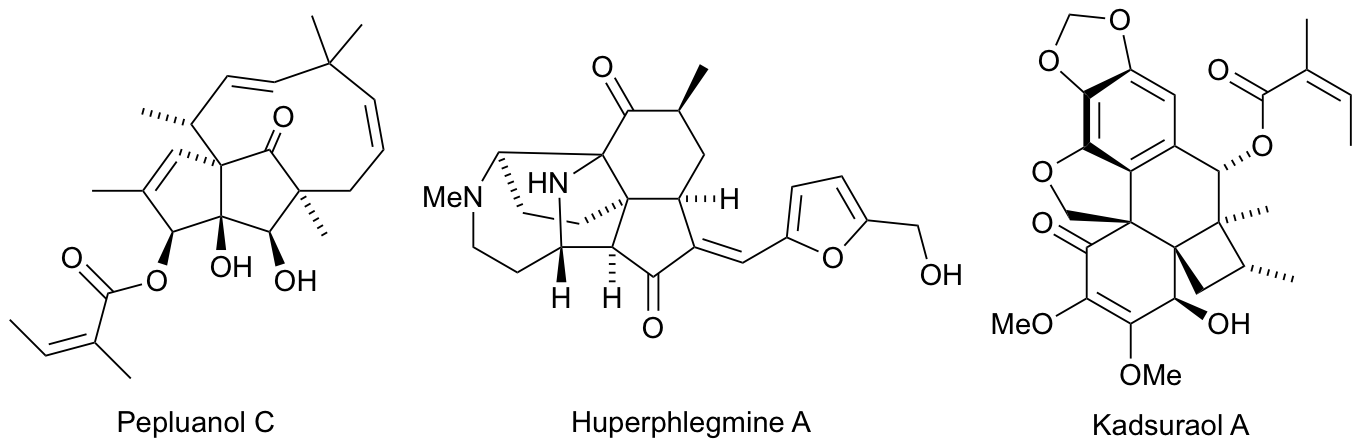
2017
N. L. Sloan, S. K. Luthra, G. McRobbie, S. L. Pimlott and A. Sutherland, Late Stage Iodination of Biologically Active Amines using a One-Pot Process from Aryl Amines, RSC Adv., 2017, 7, 54881-54891. DOI:10.1039/c7ra11860k. Highlighted in Synfacts (2018, 14, 321).
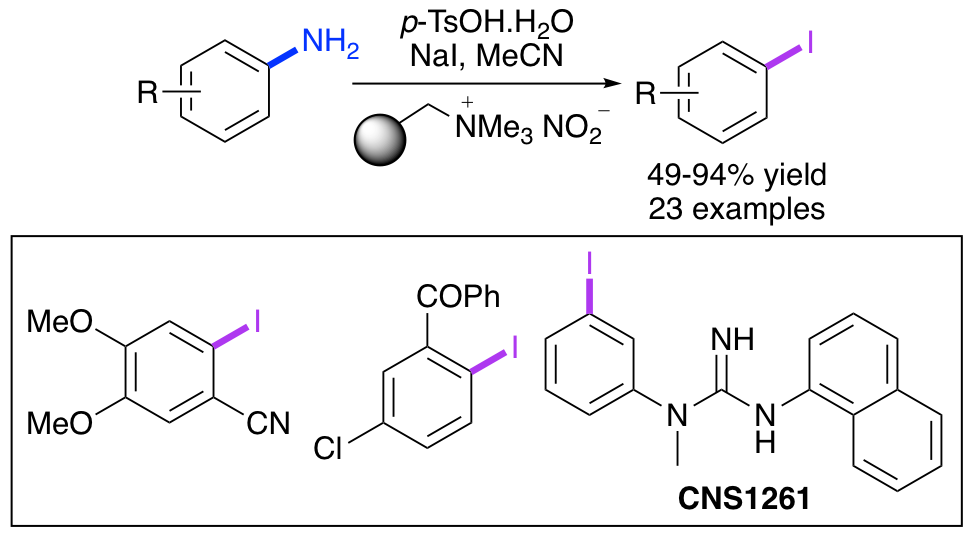
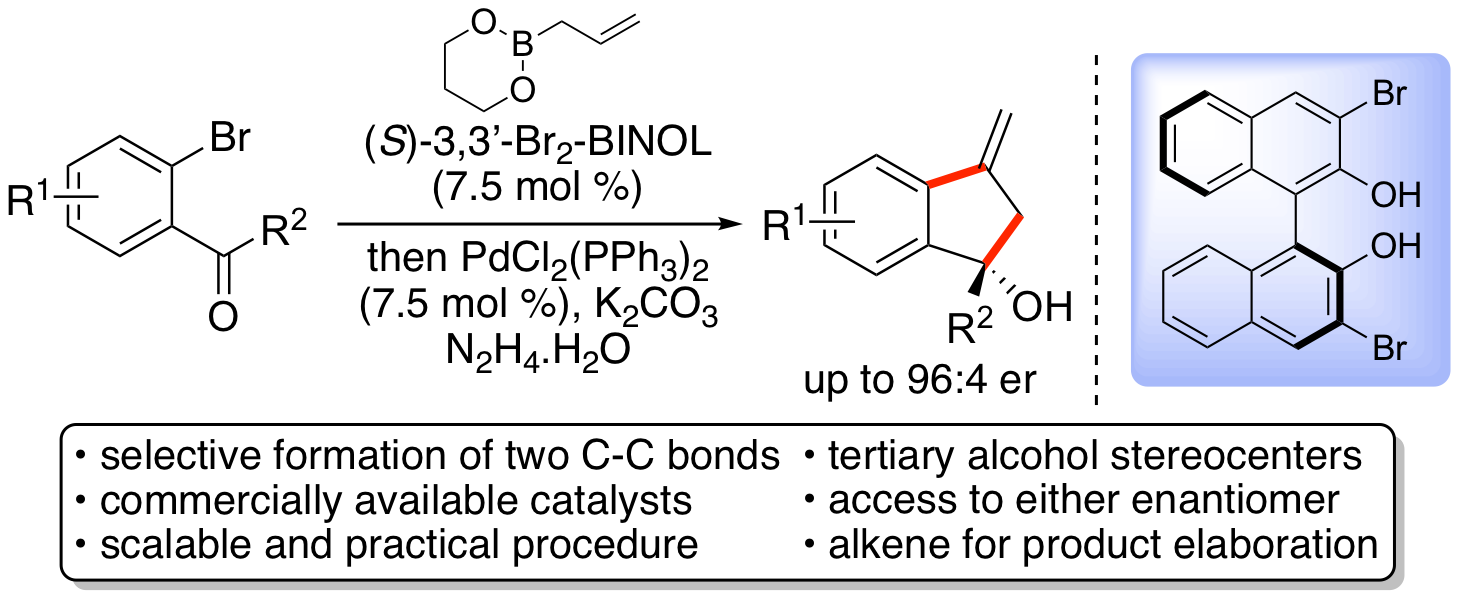
R. J. Faggyas, E. D. D. Calder, C. Wilson and A. Sutherland, One-Pot Asymmetric Synthesis of Alkylidene 1-Alkylindan-1-ols using Brønsted Acid and Palladium Catalysis, J. Org. Chem., 2017, 82, 11585-11593. DOI:10.1021/acs.joc.7b02287.
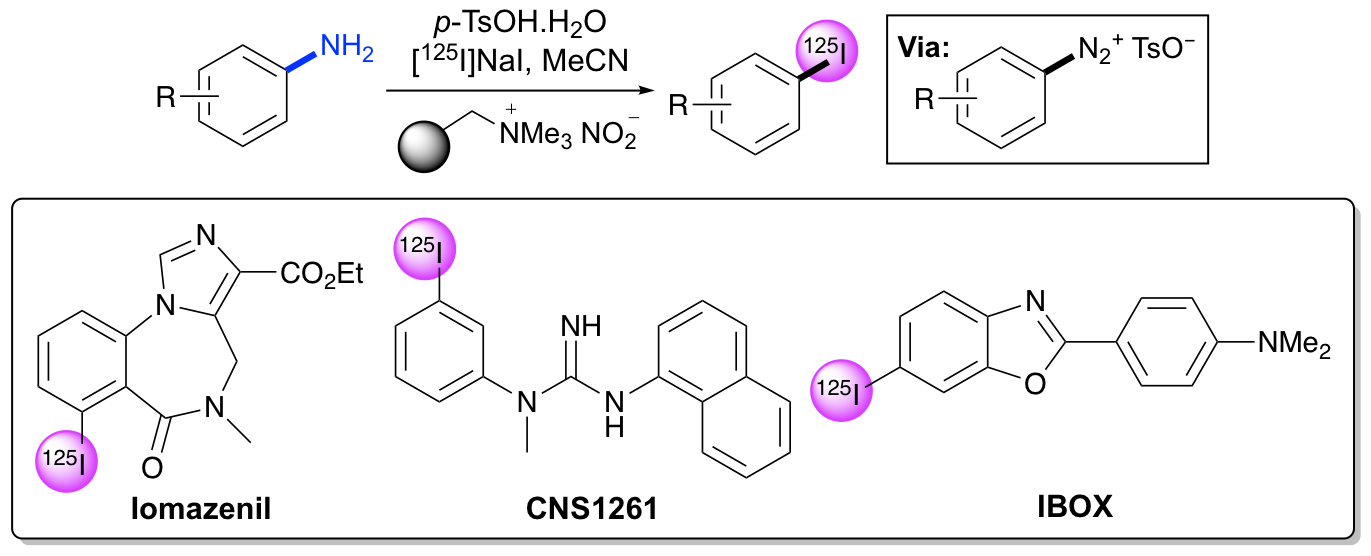
N. L. Sloan, S. K. Luthra, G. McRobbie, S. L. Pimlott and A. Sutherland, A One-Pot Radioiodination of Aryl Amines via Stable Diazonium Salts: Preparation of 125I-Imaging Agents, Chem. Commun., 2017, 53, 11008-11011. DOI:10.1039/C7CC06211G.
M. C. Henry, M. A. B. Mostafa and A. Sutherland, Recent Advances in Transition-Metal-Catalyzed, Directed Aryl C-H/N-H Cross-Coupling Reactions, Synthesis, 2017, 49, 4586-4598. DOI:10.1055/s-0036-1588536.. (Invited Submission).

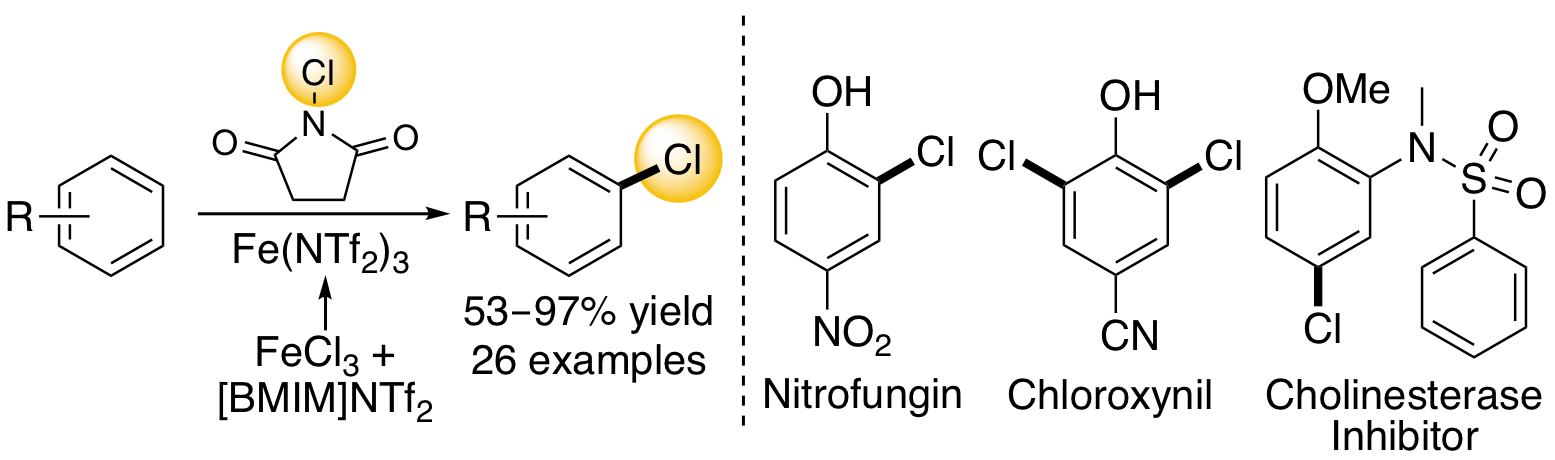
M. A. B. Mostafa, R. M. Bowley, D. T. Racys, M. C. Henry and A. Sutherland, Iron(III)-Catalyzed Chlorination of Activated Arenes, J. Org. Chem. 2017, 82, 7529-7537. DOI:10.1021/acs.joc.7b01225.
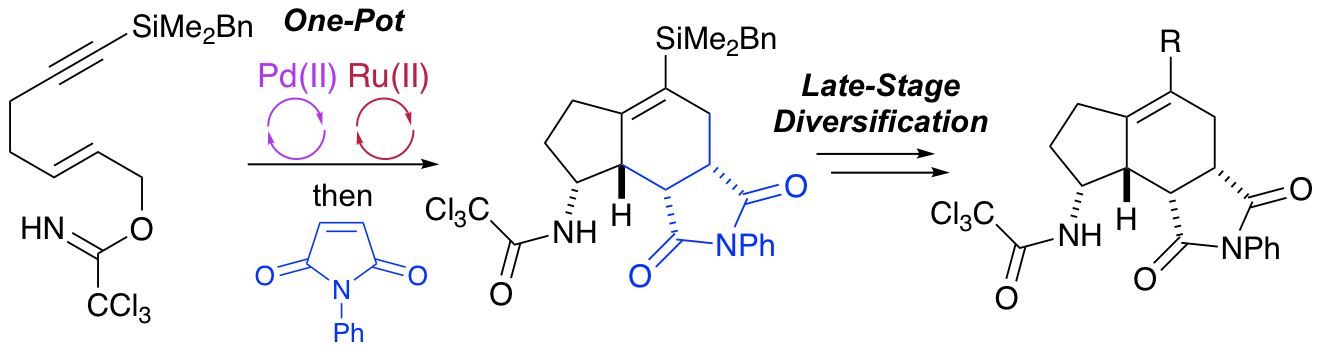
M. A. B. Mostafa, A. E. McMillan and A. Sutherland, Structural Diversification of the Aminobicyclo[4.3.0]nonane Skeleton Using Alkynylsilyl-derived Allylic Trichloroacetimidates, Org. Biomol. Chem., 2017, 15, 3035-3045. DOI:10.1039/C7OB00456G.
M. A. B. Mostafa, E. D. D. Calder, D. T. Racys and A. Sutherland, Intermolecular Aryl C-H Amination Using Sequential Iron and Copper Catalysis, Chem. Eur. J., 2017, 23, 1044-1047. DOI:10.1002/chem.201605671.
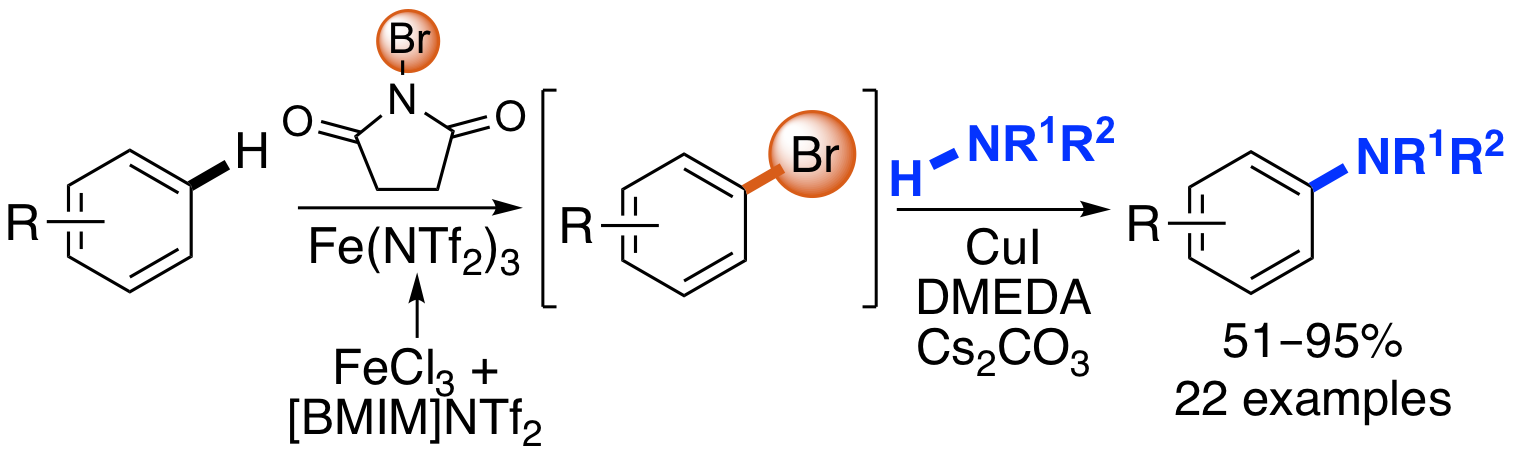
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2017, 34, 130-134, 338-342, 566-570, 940-944, 1180-1184, 1340-1344.
2016
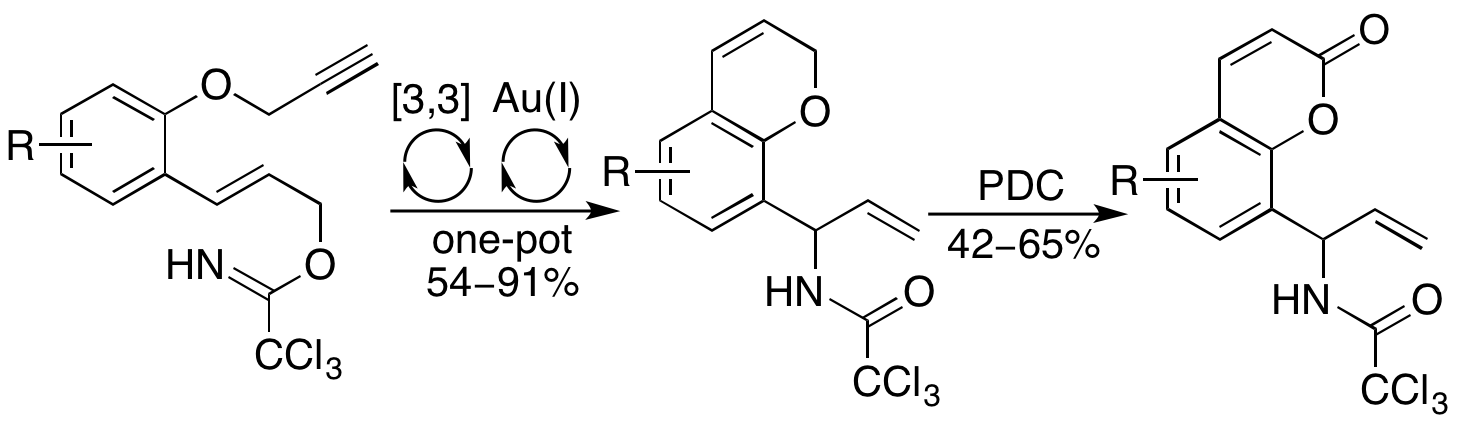
S. A. I. Sharif, E. D. D. Calder, A. H. Harkiss, M. Maduro and A. Sutherland, Synthesis of Allylic Amide Functionalized 2H-Chromenes and Coumarins Using a One-Pot Overman Rearrangement and Gold(I)-Catalyzed Hydroarylation, J. Org. Chem., 2016, 81, 9810-9819. DOI: 10.1021/acs.joc.6b01881.
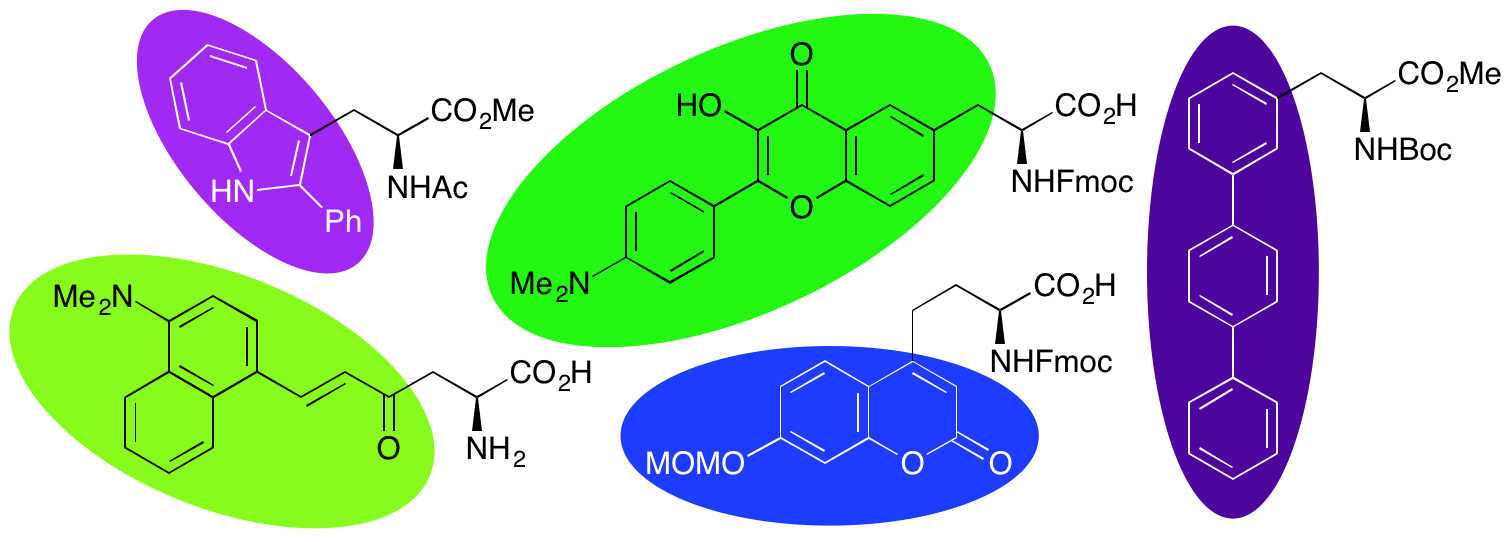
A. H. Harkiss and A. Sutherland, Recent Advances in the Synthesis and Application of Fluorescent α-Amino Acids, Org. Biomol. Chem., 2016, 14, 8911-8921. DOI:10.1039/C6OB01715K. (Invited Submission).
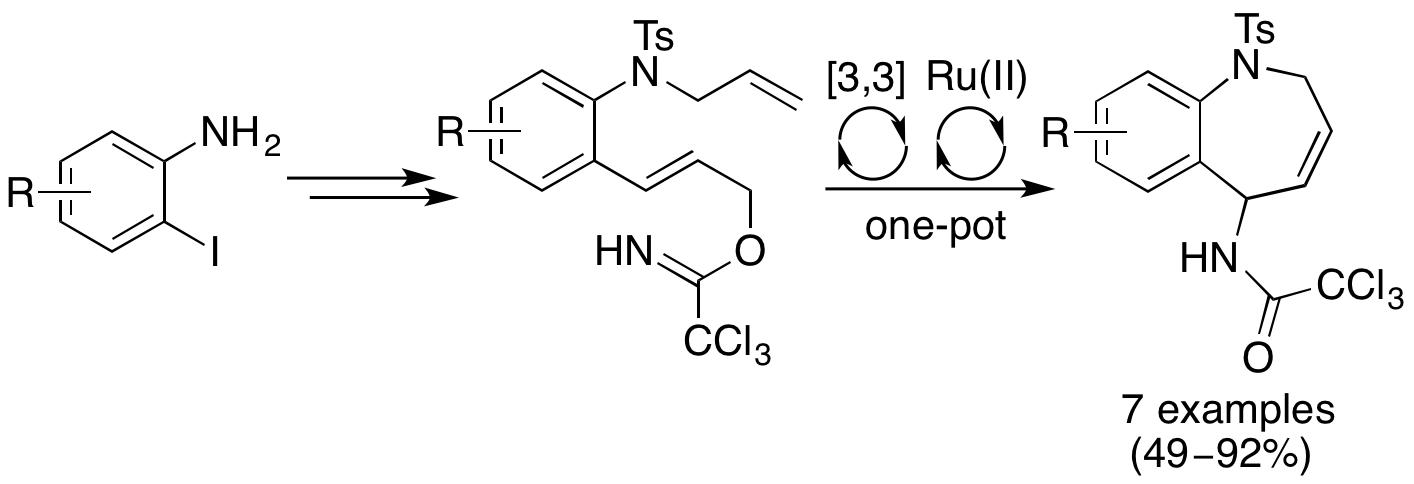
S. A. I. Sharif, E. D. D. Calder, F. G. Delolo and A. Sutherland, Synthesis of 5-Amino-2,5-dihydro-1H-benzo[b]azepines Using a One-Pot Multibond Forming Process, J. Org. Chem., 2016, 81, 6697-6706. DOI:10.1021/acs.joc.6b01357.
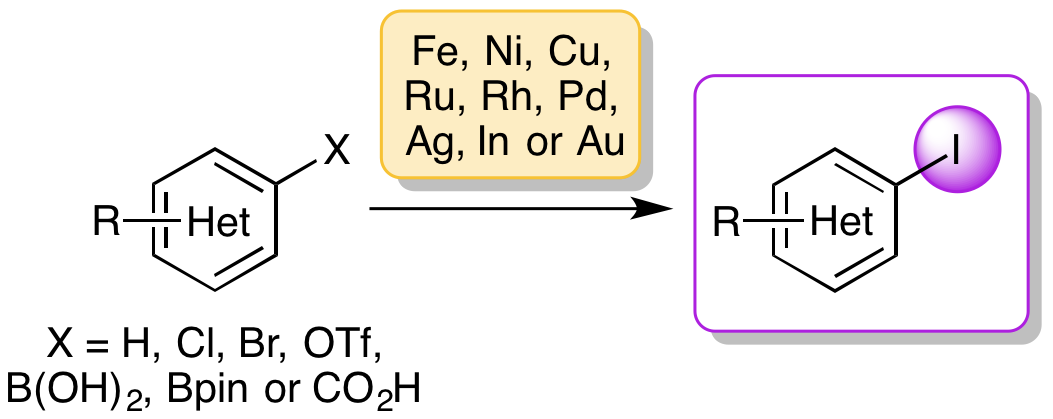
N. L. Sloan and A. Sutherland, Recent Advances in Transition-Metal-Catalyzed Iodination of Arenes, Synthesis, 2016, 48, 2969-2980. DOI:10.1055/s-0035-1562439. (Invited Submission).
M. A. B. Mostafa, M. W. Grafton, C. Wilson and A. Sutherland, A One-Pot, Three-Step Process for the Diastereoselective Synthesis of Aminobicyclo[4.3.0]nonanes using Consecutive Palladium(II)- and Ruthenium(II)-Catalysis, Org. Biomol. Chem., 2016, 14, 3284-3297. DOI:10.1039/C6OB00165C.
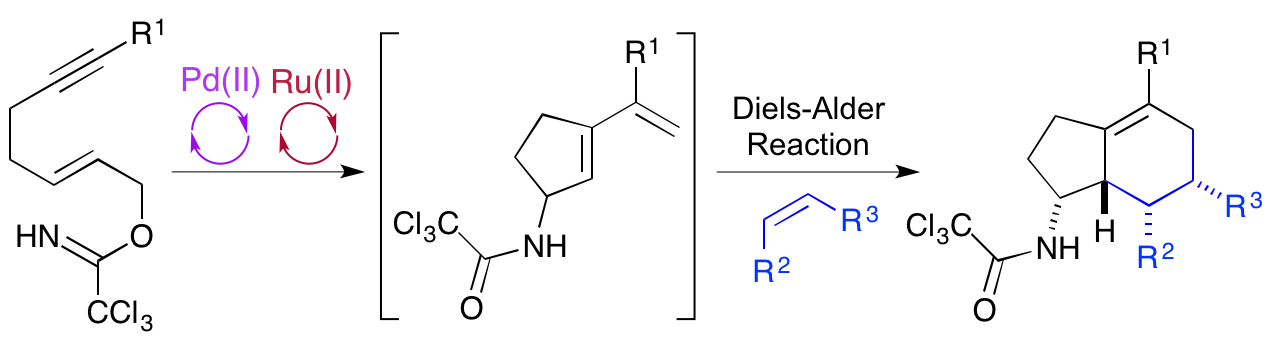
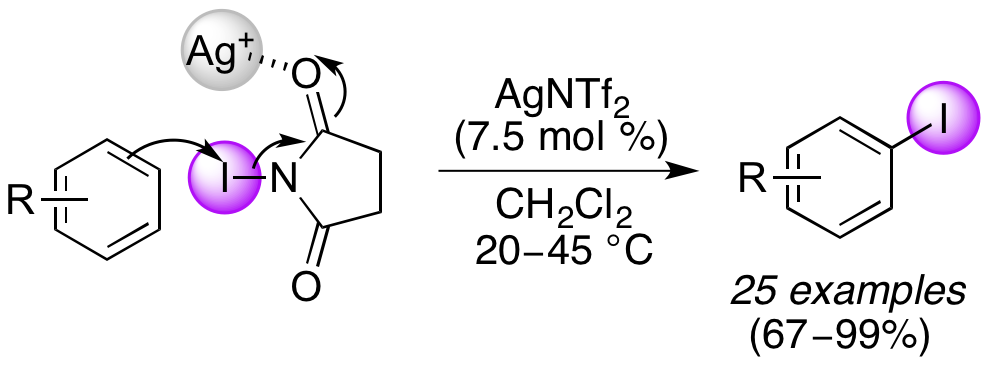
D. T. Racys, S. A. I. Sharif, S. L. Pimlott and A. Sutherland, Silver(I)-Catalyzed Iodination of Arenes: Tuning the Lewis Acidity of N-Iodosucinimide Activation, J. Org. Chem., 2016, 81, 772-780. DOI:10.1021/acs.joc.5b02761. Featured Article. (Highlighted in the Organic Chemistry Portal).
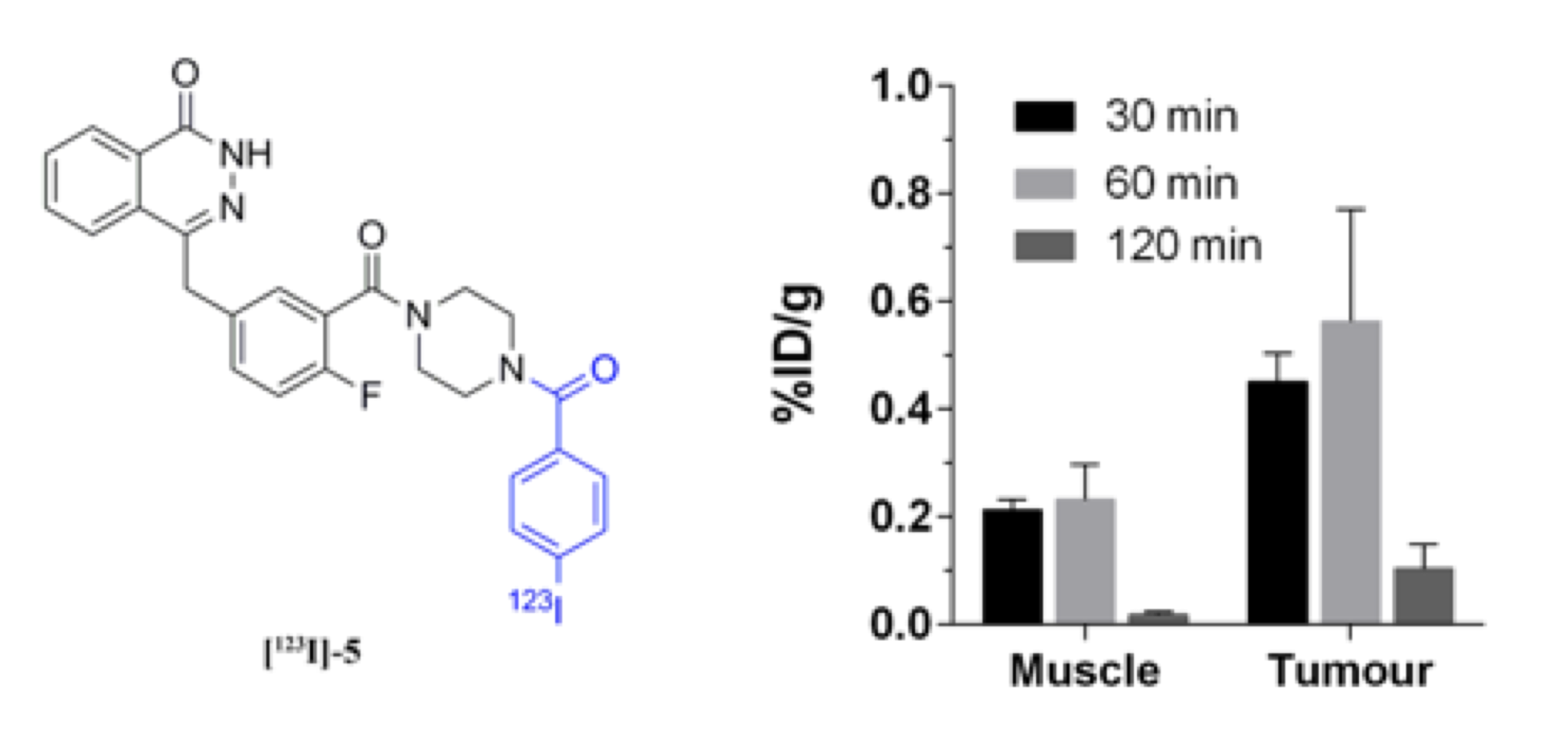
F. Zmuda, G. Malviya, A. Blair, M. Boyd, A. J. Chalmers, A. Sutherland and S. L. Pimlott, Synthesis and Evaluation of a Radioiodinated Tracer with Specificity for Poly(ADP-ribose)polymerase-1 (PARP-1) in Vivo, J. Med. Chem., 2015, 58, 8683-8693. DOI:10.1021/acs.jmedchem.5b01324.
D. T. Racys, C. E. Warrilow, S. L. Pimlott and A. Sutherland, Highly Regioselective Iodination of Arenes via Iron(III)-Catalyzed Activation of N-Iodosuccinimide, Org. Lett., 2015, 17, 4782-4785. DOI:10.1021/acs.orglett.5b02345. (Highlighted in the Organic Chemistry Portal).
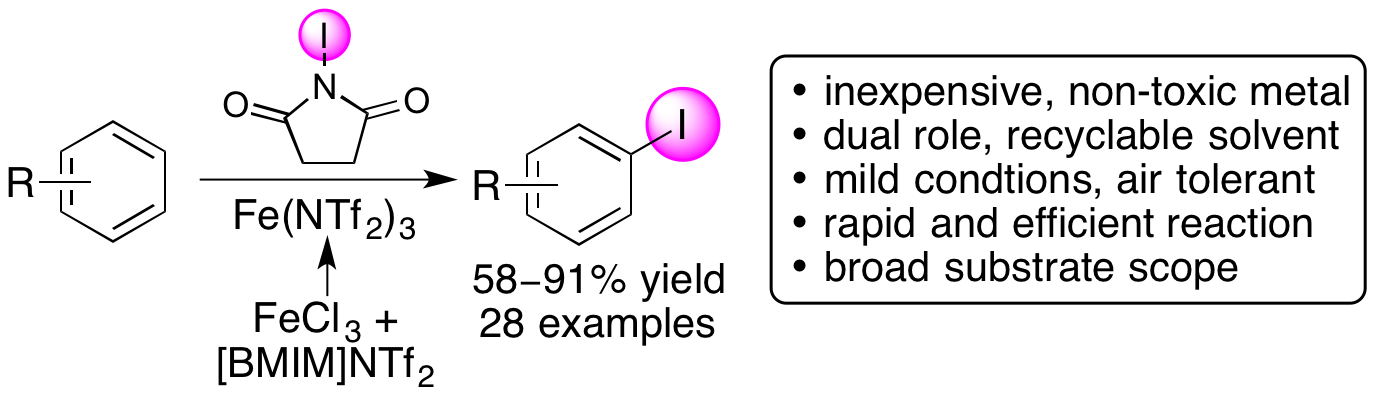
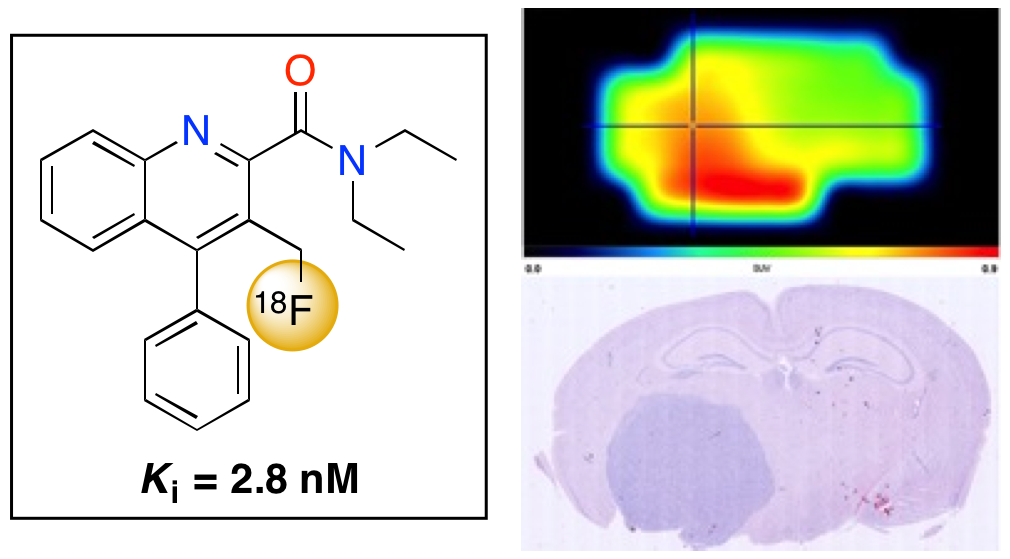
A. Blair, F. Zmuda, G. Malviya, A. A. S. Tavares, G. D. Tamagnan, A. J. Chalmers, D. Dewar, S. L. Pimlott and A. Sutherland, A Novel 18F-Labelled High Affinity Agent for PET Imaging of the Translocator Protein, Chem. Sci., 2015, 6, 4772-4777. DOI:10.1039/C5SC01647A. Highlighted in Chemistry and Industry.
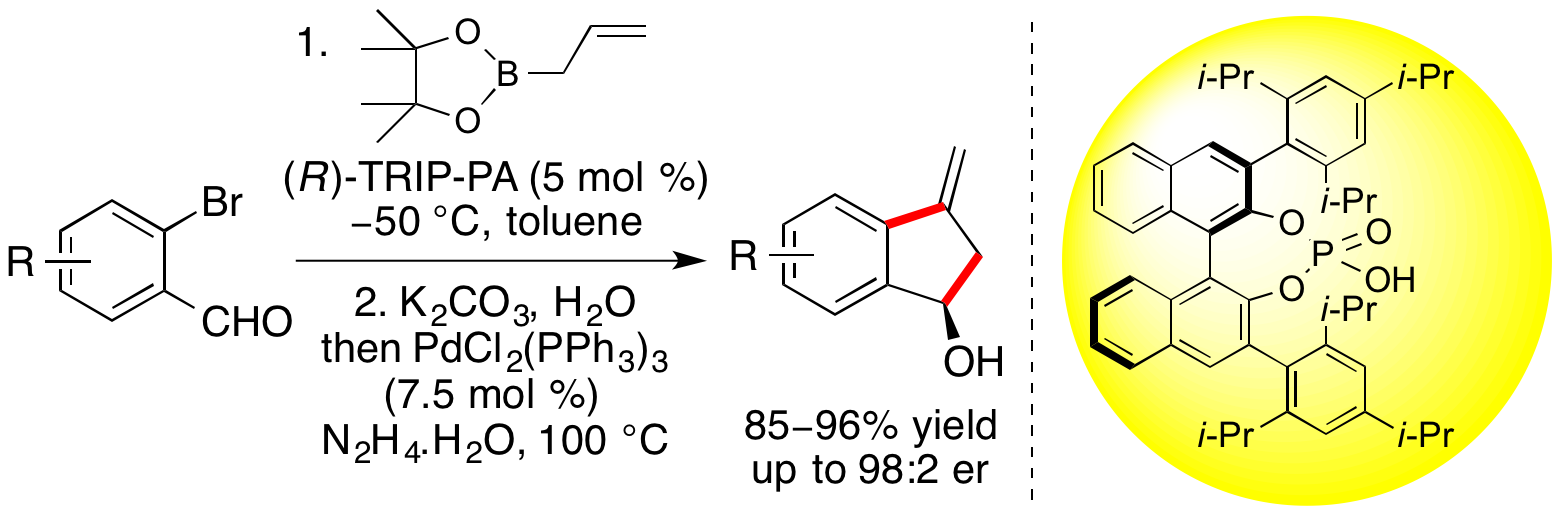
E. D. D. Calder and A. Sutherland, Enantioselective Synthesis of 3-Methyleneindan-1-ols via a One-Pot Allylboration-Heck Reaction of 2-Bromobenzaldehydes, Org. Lett., 2015, 17, 2514-2517. DOI:10.1021/acs.orglett.5b01047.
E. D. D. Calder, S. A. I. Sharif, F. I. McGonagle and A. Sutherland, One-Pot Synthesis of 5-Amino-2,5-Dihydro-1-Benzoxepines: Access to Pharmacologically Active Heterocyclic Scaffolds, J. Org. Chem., 2015, 80, 4683-4696. DOI:10.1021/acs.joc.5b00583.
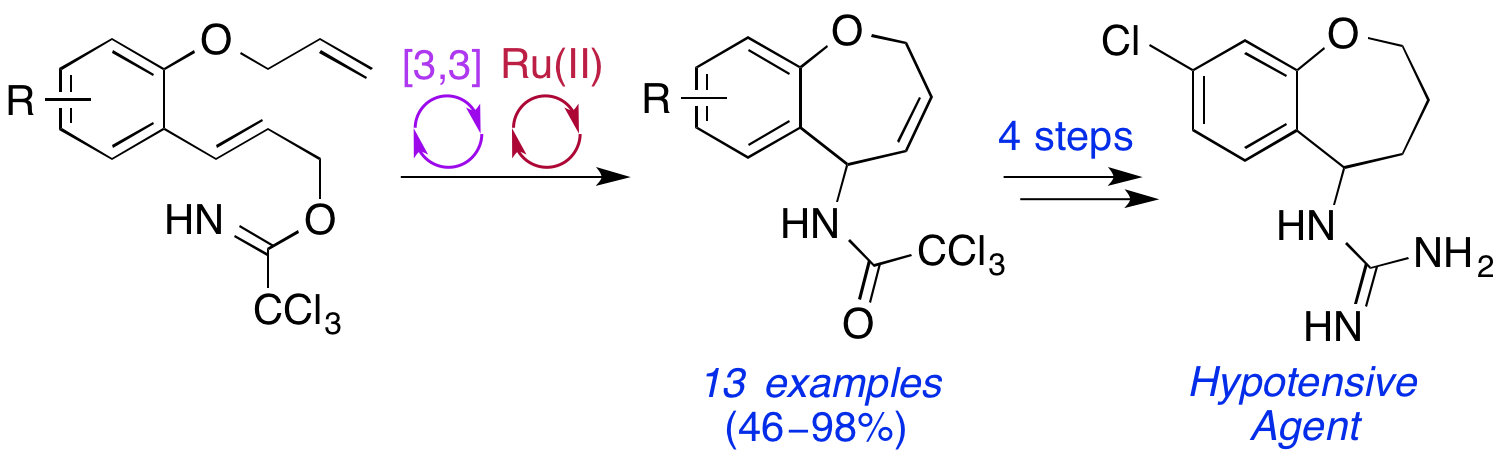
L. Gilfillan, R. Artschwager, A. H. Harkiss, R. M. J. Liskamp and A. Sutherland, Synthesis of Pyrazole Containing α-Amino Acids via a Highly Regioselective Condensation/Aza-Michael Reaction of β-Aryl α,β-Unsaturated Ketones, Org. Biomol. Chem., 2015, 13, 4514-4523. DOI:10.1039/C5OB00364D.
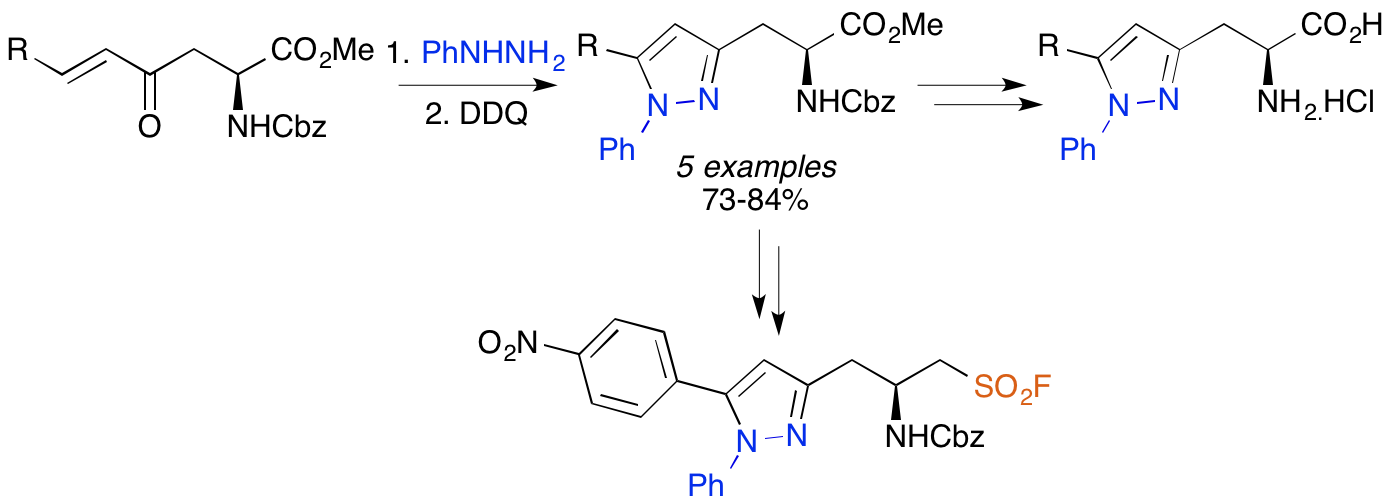
C. M. Reid, K. N. Fanning, L. S. Fowler and A. Sutherland, Synthesis and Reactivity of 4-Oxo-5-trimethylsilanyl Derived α-Amino Acids, Tetrahedron, 2015, 71, 245-251. DOI:10.1016/j.tet.2014.11.059.
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2015, 32, 111-115, 512-516, 760-764, 1165-1169, 1364-1368, 1612-1616.
2014
E. D. D. Calder, F. I. McGonagle, A. H. Harkiss, G. A. McGonagle and A. Sutherland, Preparation of Amino-Substituted Indenes and 1,4-Dihydronaphthalenes Using a One-Pot Multi-Reaction Approach: Total Synthesis of Oxybenzo[c]phenanthridine Alkaloids, J. Org. Chem., 2014, 79, 7633-7648. DOI:10.1021/jo5014492.

M. W. Grafton, S. A. Johnson, L. J. Farrugia and A. Sutherland, Diastereoselective Synthesis of Highly Substituted Polycyclic Scaffolds via a One-Pot Four-Step Tandem Catalytic Process, Tetrahedron, 2014, 70, 7133-7141. DOI:10.1016/j.tet.2014.06.020. Special issue in memory of Professor Sandy McKillop.
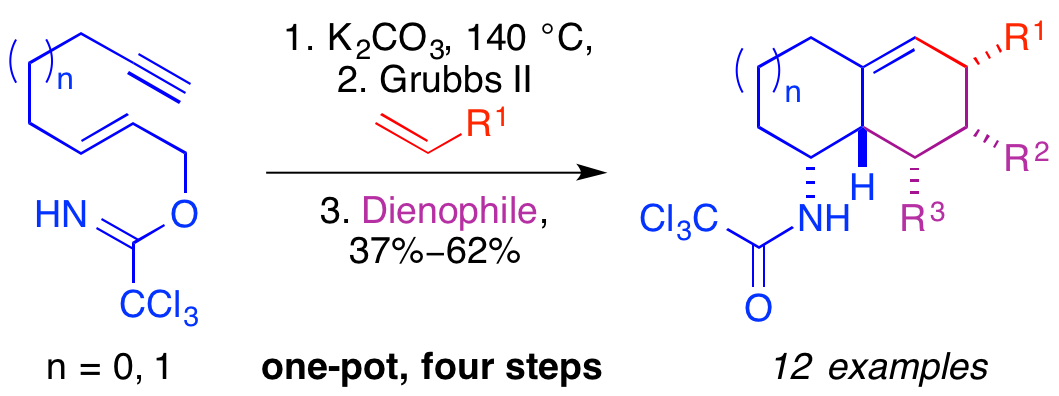
E. D. D. Calder, M. W. Grafton and A. Sutherland, One-Pot Multi-Reaction Processes: Synthesis of Natural Products and Drug-Like Scaffolds, Synlett, 2014, 25, 1068-1080. DOI:10.1055/s-0033-1340683. Research Account.
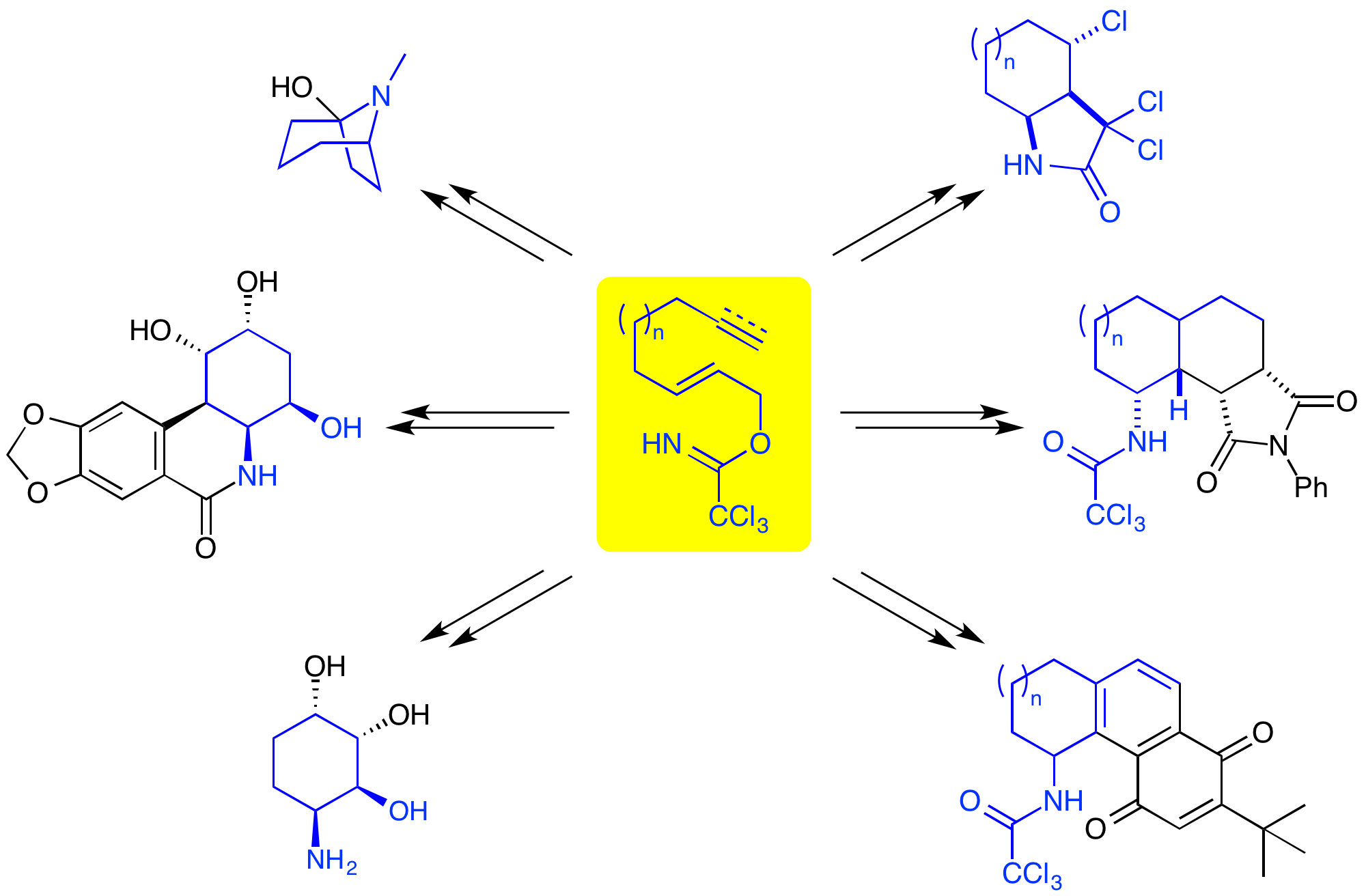
A. M. Zaed, M. W. Grafton, S. Ahmad and A. Sutherland, Asymmetric Synthesis of cis-Aminocyclopentenols, Building Blocks for Medicinal Chemistry, J. Org. Chem., 2014, 79, 1511-1515. DOI:10.1021/jo402712r.
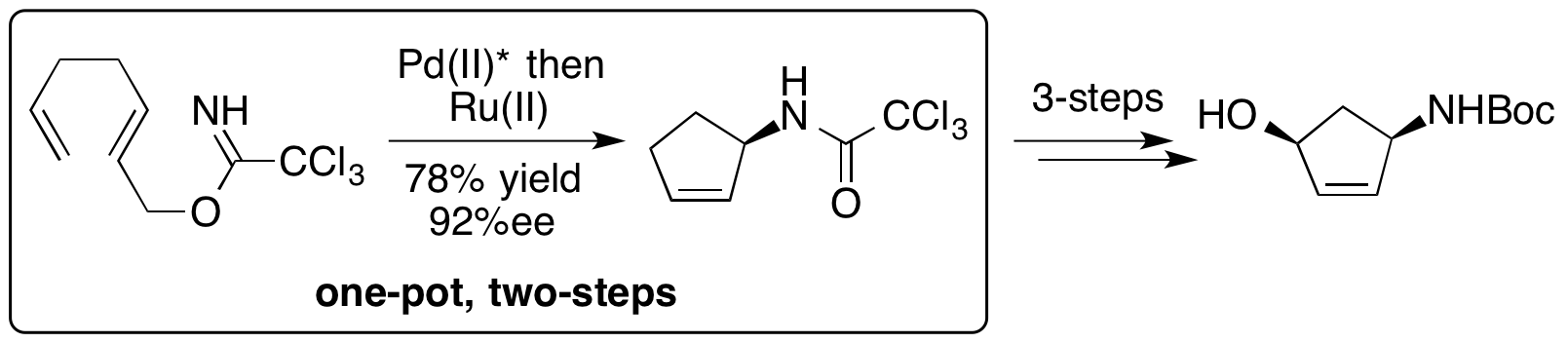
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2014, 31, 148-153, 414-418, 706-710, 1083-1087, 1242-1246, 1671-1675.
2013
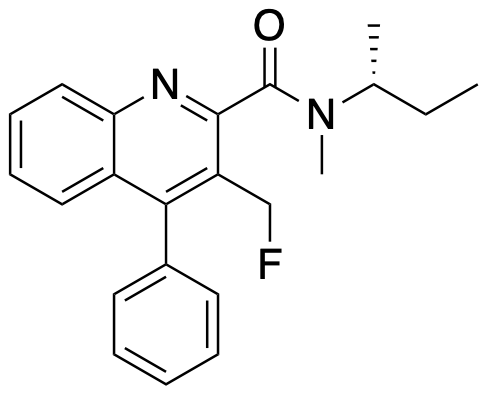
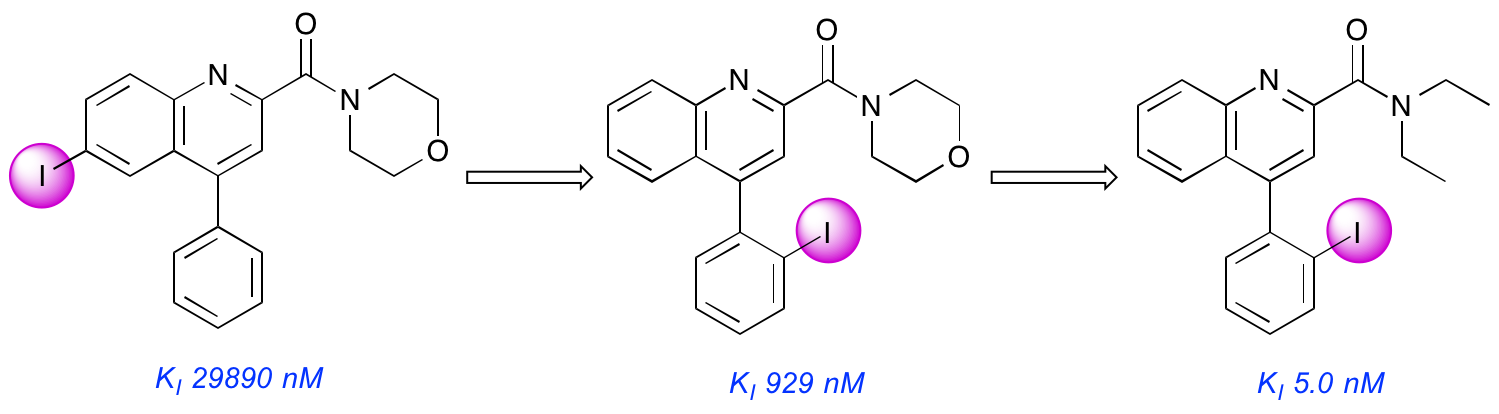
A. Blair, L. Stevenson, D. Dewar, S. L. Pimlott and A. Sutherland, Structure-Activity Relationships of Novel Iodinated Quinoline-2-carboxamides for Targeting the Translocator Protein, Med. Chem. Commun, 2013, 4, 1461-1466. DOI: 10.1039/C3MD00249G.
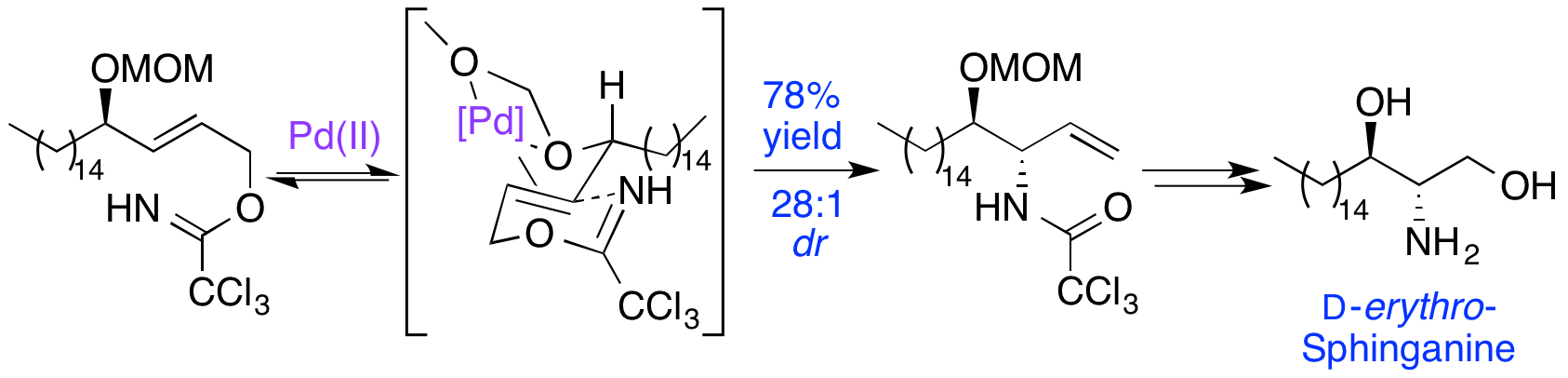
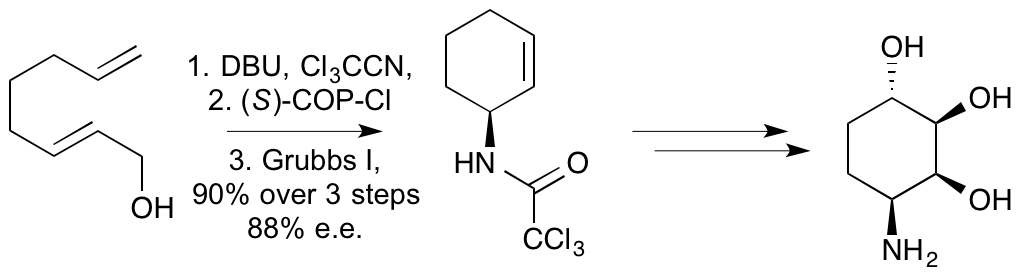
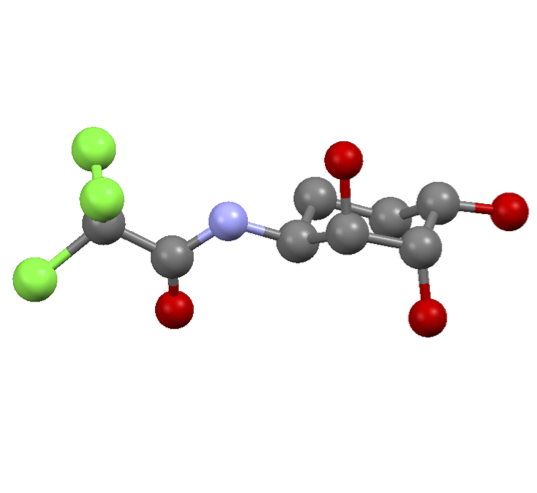
E. D. D. Calder, A. M. Zaed and A. Sutherland, Preparation of anti-Vicinal Amino Alcohols: Asymmetric Synthesis of D-erythyro-Sphinganine, (+)-Spisulosine and D-ribo-Phytosphingosine, J. Org. Chem., 2013, 78, 7223-7233. DOI:10.1021/jo401211j.
M. W. Grafton, L. J. Farrugia and A. Sutherland, Synthesis of Substituted Indanes and Tetralins via Consecutive Multi-Bond Forming Tandem Processes, J. Org. Chem., 2013, 78, 7199-7207. DOI:10.1021/jo401182r
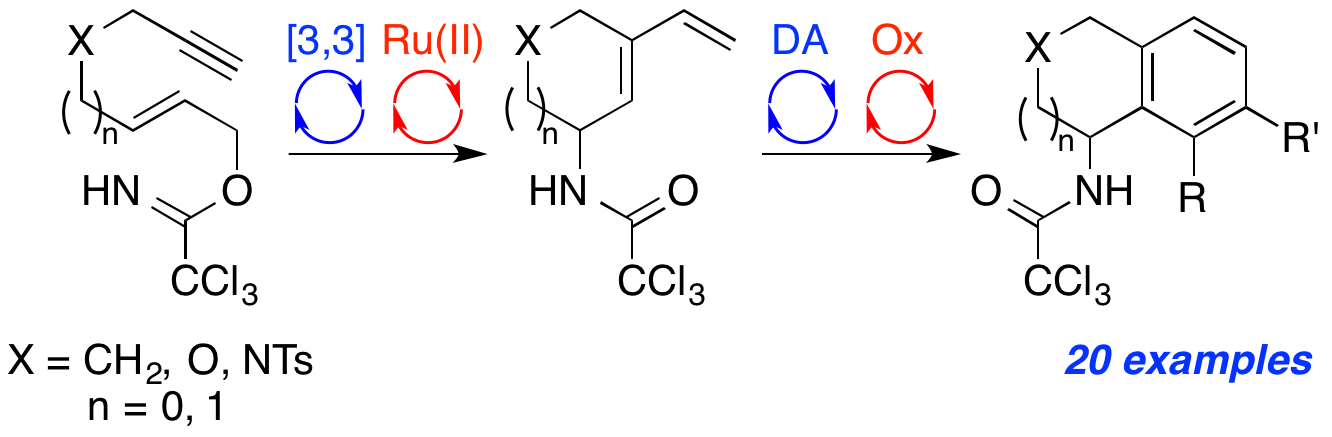 |
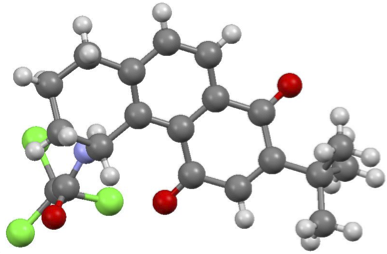 |
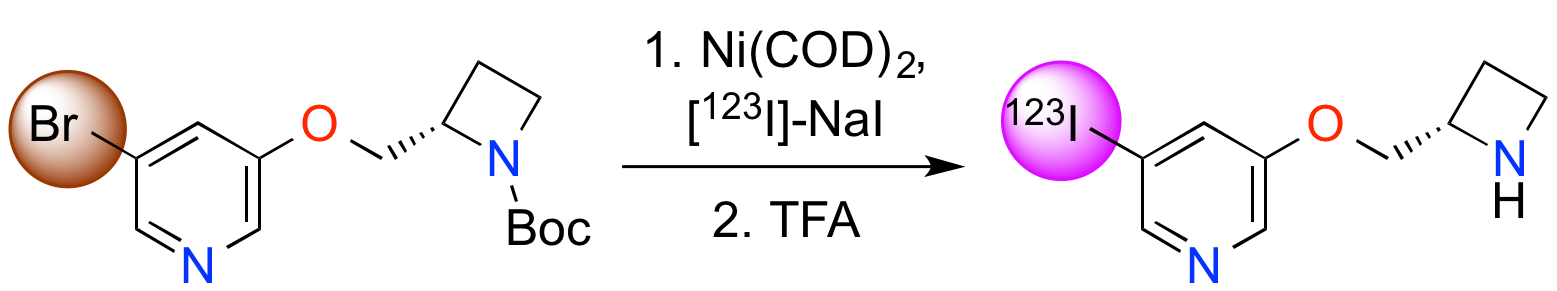
A. A. Cant, S. Champion, R. Bhalla, S. L. Pimlott and A. Sutherland, Nickel-Mediated Radioiodination of Aryl and Heteroaryl Bromides: Rapid Synthesis of Tracers for SPECT Imaging, Angew. Chem. Int. Ed., 2013, 52, 7829-7832. DOI: 10.1002/anie.201302800
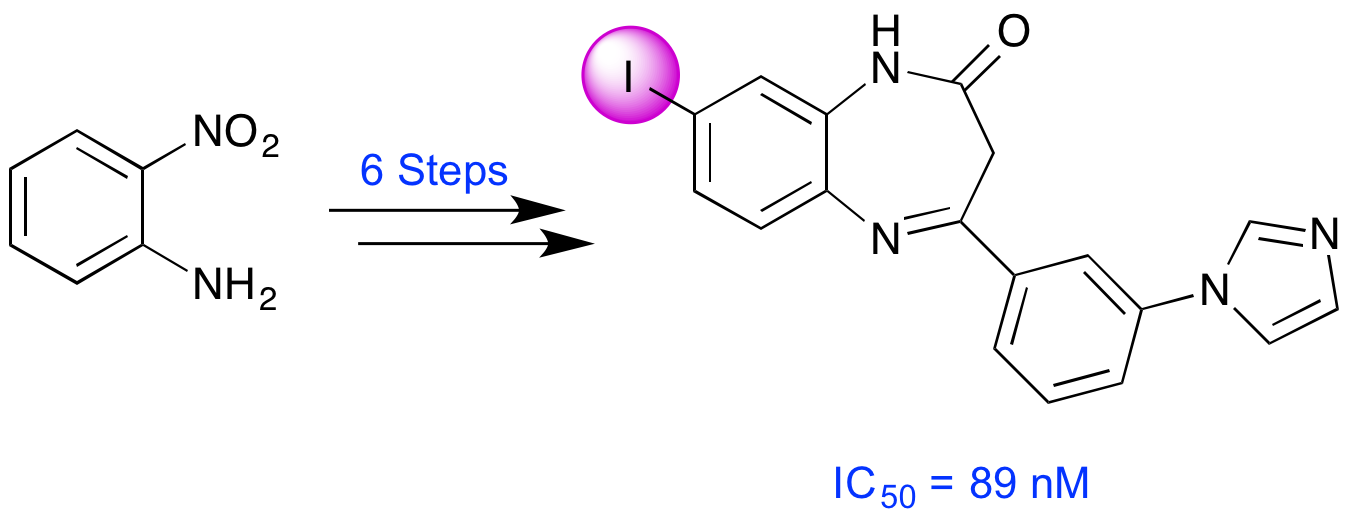
L. Gilfillan, A. Blair, B. J. Morris, J. A. Pratt, L. Schweiger, S. Pimlott and A. Sutherland, Synthesis and Biological Evaluation of Novel 2,3-Dihydro-1H-1,5-benzodiazepin-2-ones; Potential Imaging Agents of the Metabotropic Glutamate 2 Receptor, Med. Chem. Commun., 2013, 4, 1118-1123. DOI: 10.1039/C3MD00110E.
R. A. Hill and A. Sutherland, Hot off the Press, Nat. Prod. Rep., 2013, 30, 213-217, 485-489, 760-764, 1074-1078, 1272-1276, 1462-1466.
2012
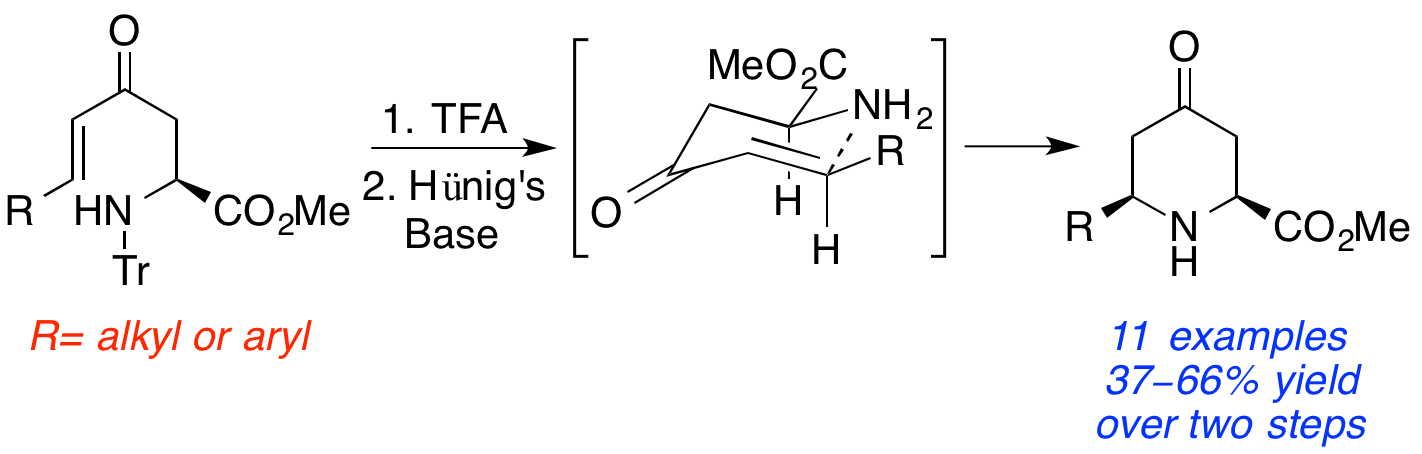
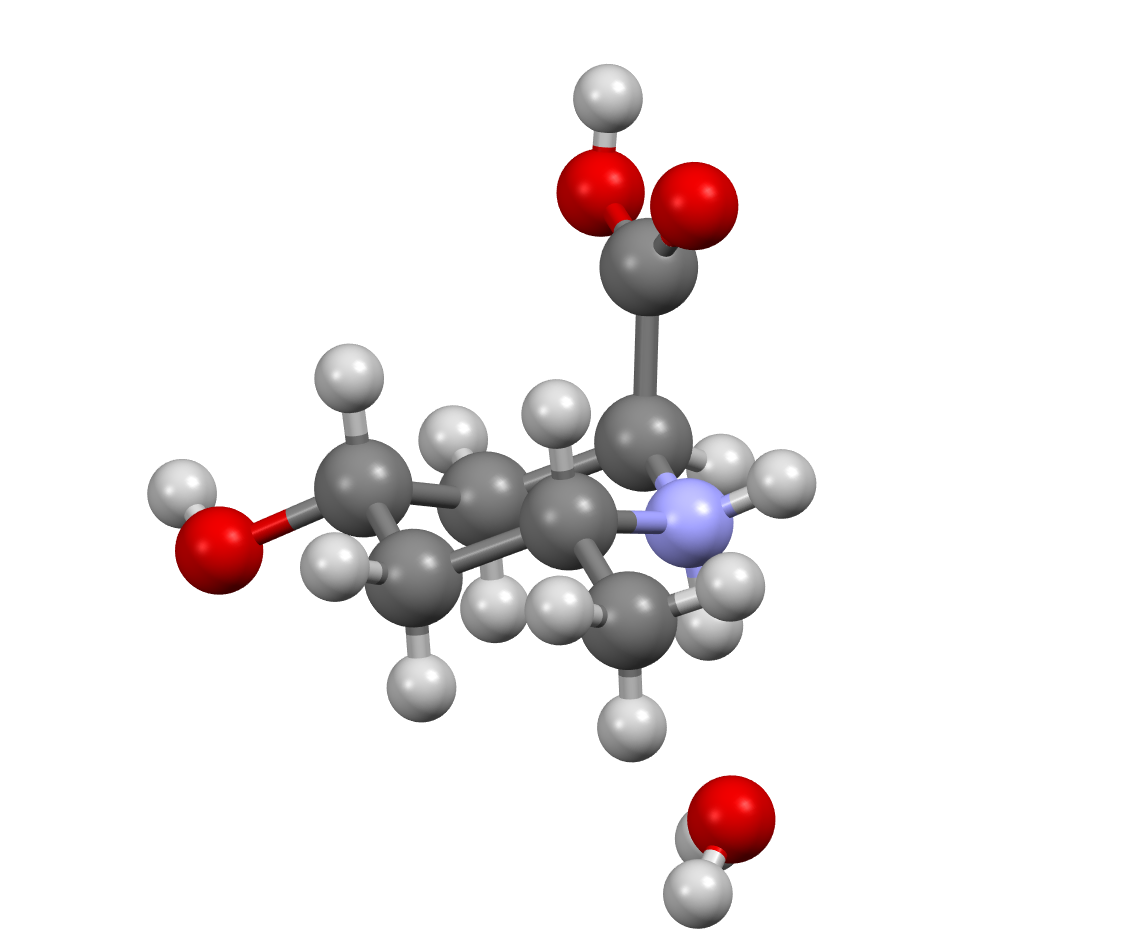
M. Daly, A. A. Cant, L. S. Fowler, G. L. Simpson, H. M. Senn and A. Sutherland, Switching the Stereochemical Outcome of 6-Endo-Trig Cyclizations; Synthesis of 2,6-Cis-6-Substituted 4-Oxopipecolic Acids, J. Org. Chem., 2012, 77, 10001-10009. Featured Article. DOI: 10.1021/jo3022583
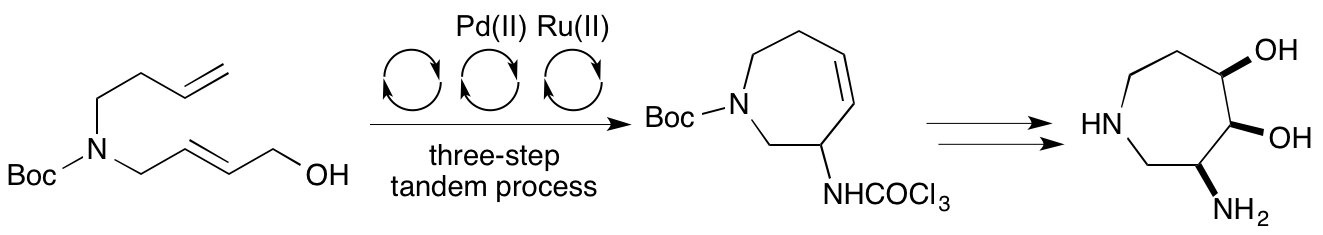
S. Ahmad and A. Sutherland, Stereoselective Synthesis of Hydroxylated 3-Aminoazepanes Using a Multi-Bond Forming, Three-Step Tandem Process, Org. Biomol. Chem., 2012, 10, 8251-8259. DOI: 10.1039/C2OB26544C
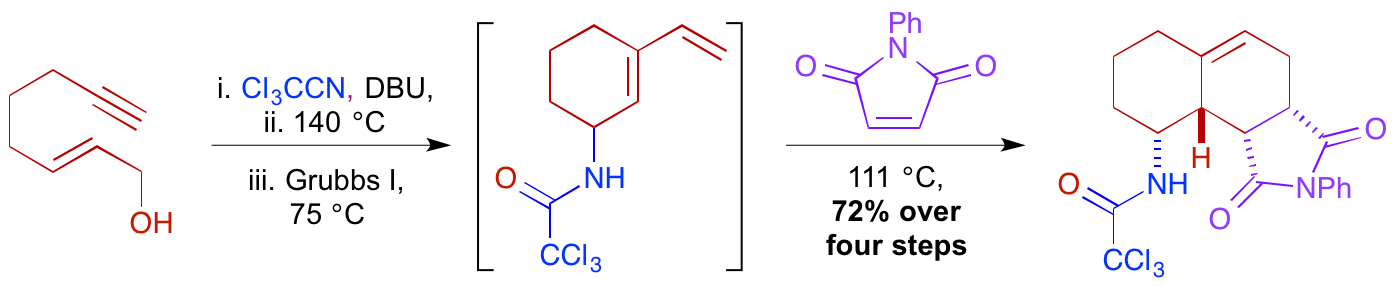
M. W. Grafton, L. J. Farrugia, H. M. Senn and A. Sutherland, Discovery Of A Multi-Bond Forming, Four-Step Tandem Process: Construction Of Drug-Like Polycyclic Scaffolds, Chem. Commun., 2012, 48, 7994-7996. DOI:10.1039/C2CC33649A
 |
 |
 |
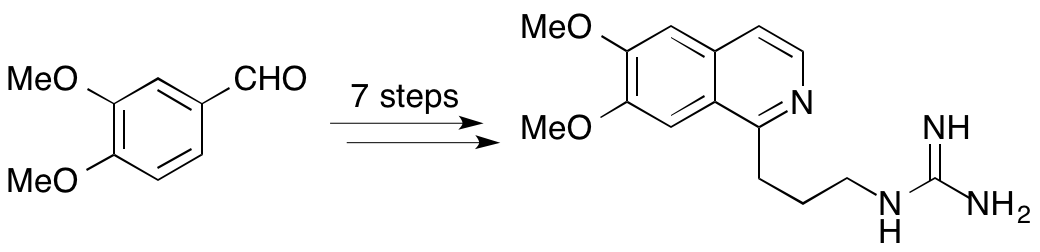
A. Blair, L. Stevenson and A. Sutherland, Synthesis Of The Isoquinoline Alkaloid, Crispine C, Tetrahedron Lett., 2012, 53, 4084-4086. DOI: j.tetlet.2012.05.113
A. A. Cant, R. Bhalla, S. L. Pimlott and A. Sutherland, Nickel-Catalysed Aromatic Finkelstein Reaction Of Aryl and Heteroaryl Bromides, Chem. Commun., 2012, 48, 3993-3995. DOI: 10.1039/C2CC30956D
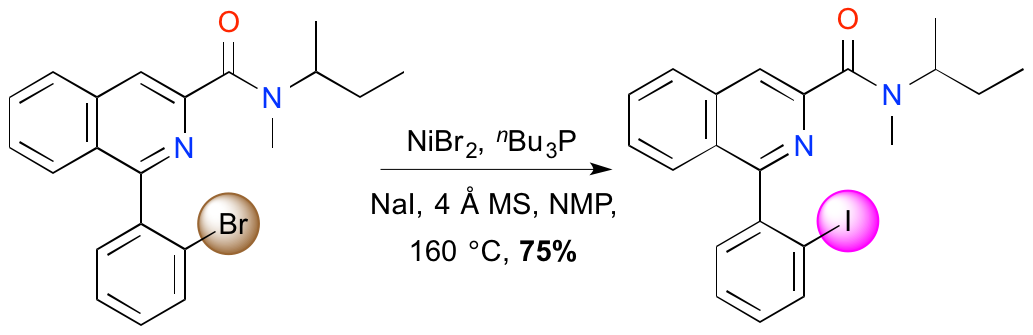
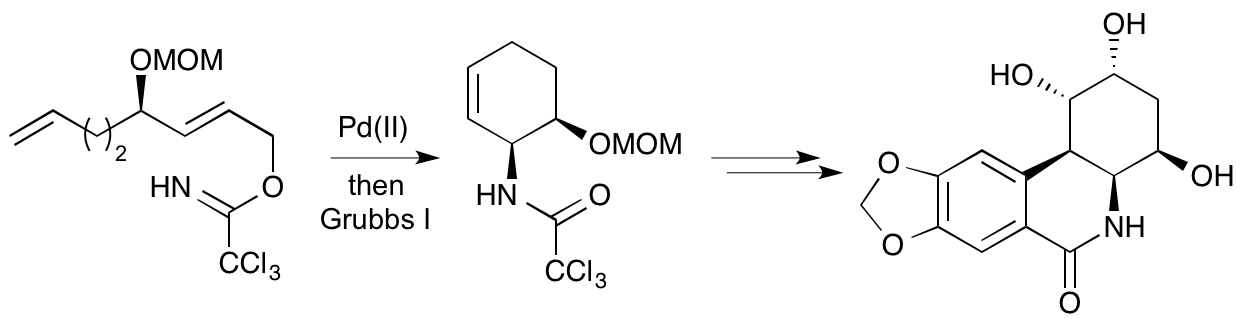
S. Ahmad, M. D. Swift, L. J. Farrugia, H. M. Senn and A. Sutherland, Stereoselective Synthesis Of Functionalised Carbocyclic Amides; Construction Of The syn-(4aS,10bS)-Phenanthridone Skeleton, Org. Biomol. Chem., 2012, 10, 3937-3945. DOI: 10.1039/C2OB25334H
|
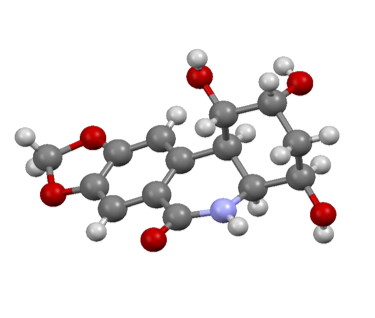 |
A. A. Cant and A. Sutherland, Asymmetric Synthesis Of Pipecolic Acid And Derivatives, Synthesis, 2012, 44, 1935-1950. Invited Review. DOI: 10.1055/s-0031-1289767
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2012, 29, 129-133, 435-439, 617-621, 829-833, 1033-1037, 1377-1381.
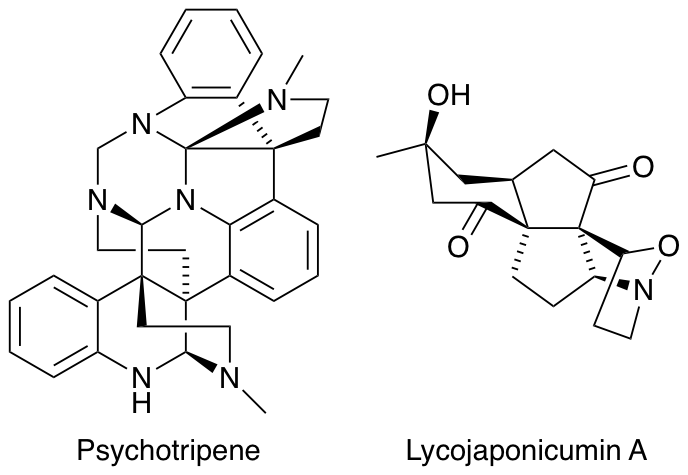
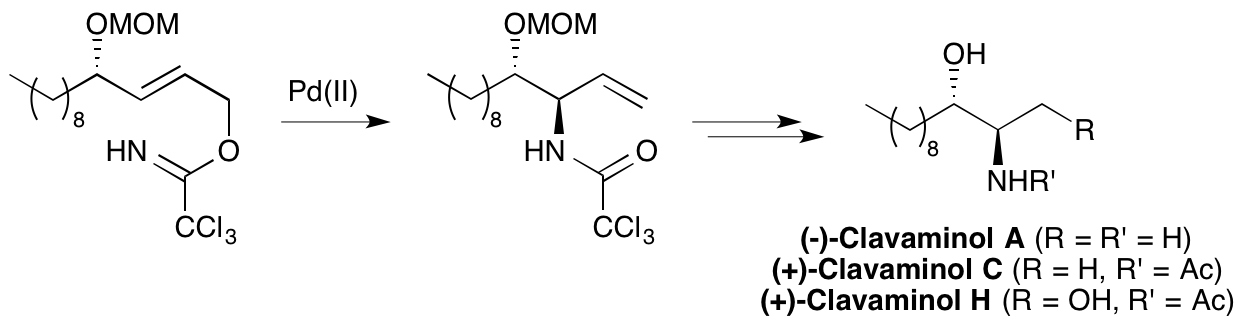
2011
A. Zaed and A. Sutherland, Total Synthesis Of Clavaminol A, C and H, Org. Biomol. Chem., 2011, 9, 8030-8037. DOI: 10.1039/C1OB06060K
 |
 |
L. S. Fowler, L. H. Thomas, D. Ellis and A. Sutherland, A One-Pot, Reductive Amination/6-endo-trig Cyclisation For The Stereoselective Synthesis Of 6-Substituted-4-Oxopipecolic Acids, Chem. Commun., 2011, 47, 6569-6571. DOI: 10.1039/C1CC11916H
 |
 |
 |
 |
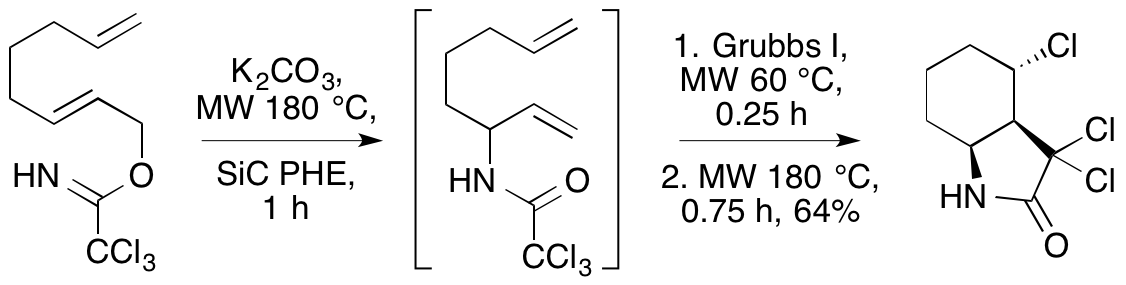
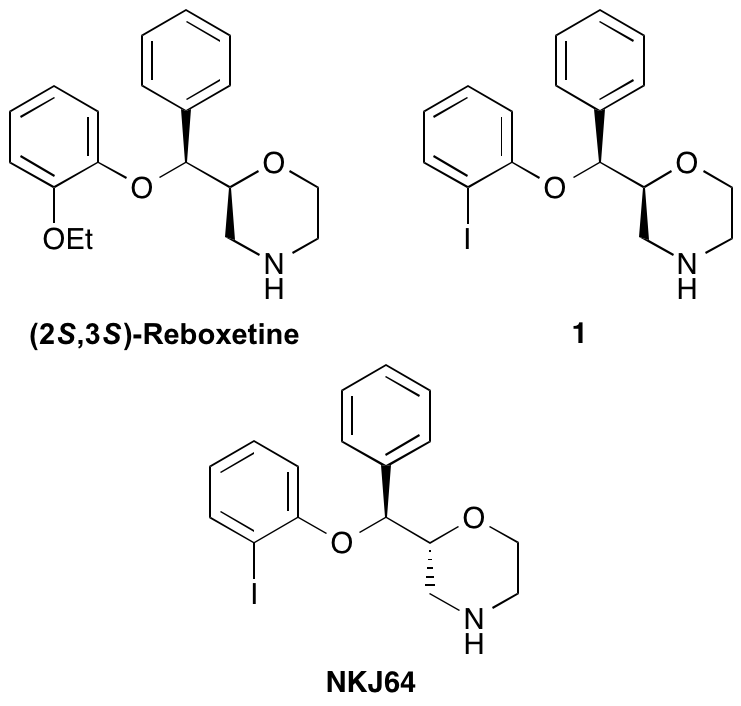
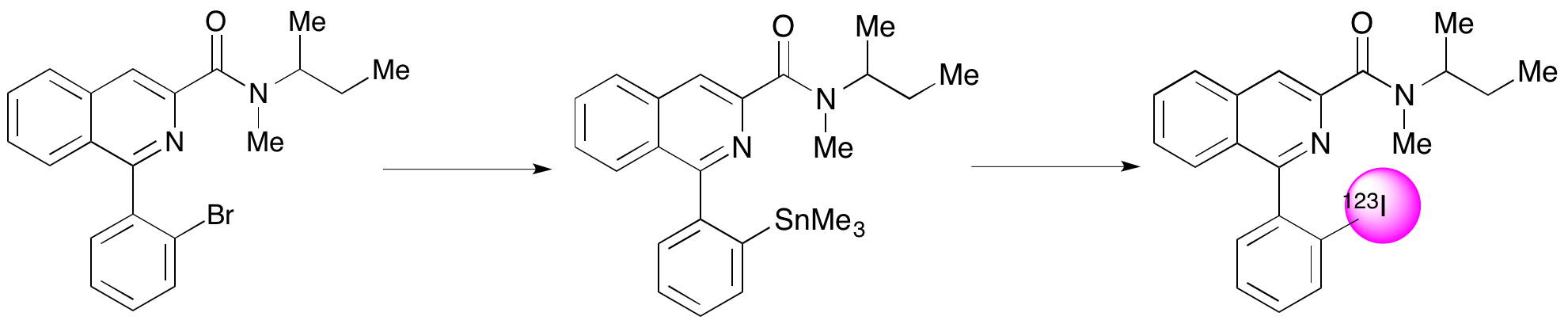
A. A. S. Tavares, N. K. Jobson, D. Dewar, A. Sutherland and S. Pimlott, 123I-NKJ64: A Novel SPECT Radiotracer For Imaging The Noradrenaline Transporter In Brain, Synapse, 2011, 65, 658-667. DOI: 10.1002/syn.20895
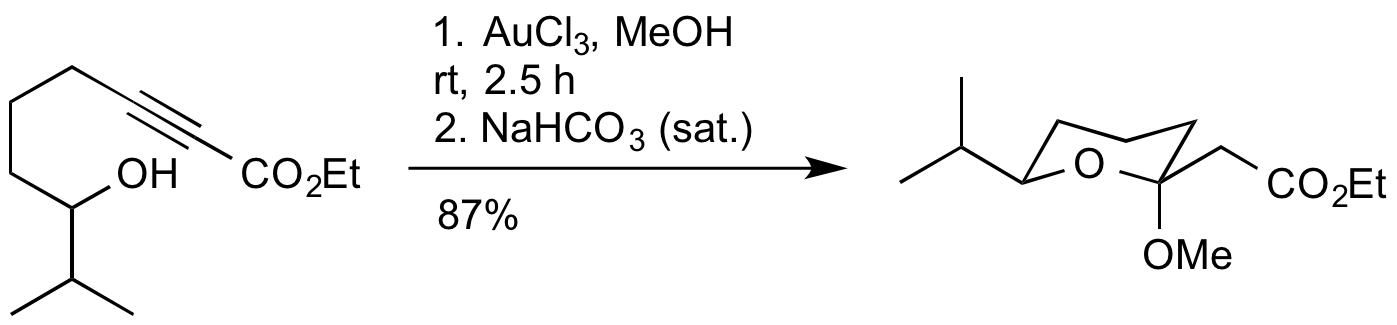
L. S. Fowler and A. Sutherland, Product Class 6: Acyclic And Semicyclic O/O Acetals, In Science Of Synthesis Knowledge Updates, D. J. Procter, G. A. Molander, Eds.; Thieme: Stuttgart, 2011, Vol. 2010/3, Section 29.6.2, p 121-150.
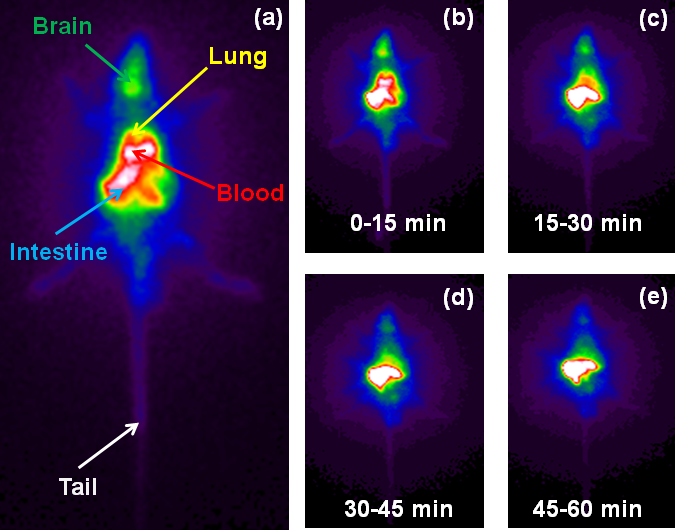
A. A. S. Tavares, N. K. Jobson, D. Dewar, A. Sutherland and S. Pimlott, Development Of The Radiosynthesis Of High-Specific-Activity 123I-NKJ64, Nucl. Med. Biol., 2011, 38, 493-500. DOI: 10.1016/j.nucmedbio.2010.11.011
S. L. Pimlott and A. Sutherland, Molecular Tracers For The PET And SPECT Imaging Of Disease, Chem. Soc. Rev., 2011, 40, 149-162. DOI: 10.1039/B922628C
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2011, 28, 182-185, 659-662, 1031-1034, 1345-1349, 1621-1625, 1879-1882.
2010
A. R. Kennedy, W. J. Kerr, L. C. Paterson and A. Sutherland, A Tetrahydropentaleno[1,6a-a]naphthalen-4(2H)-one Of Defined Relative Stereochemistry For Use Towards Agariblazeispirol C, Acta Cryst., 2010, C66, o473-o474. DOI: 10.1107/S0108270110031781

A. M. Zaed and A. Sutherland, Stereoselective Synthesis Of The Bicyclic Guanidine Alkaloid (+)-Monanchorin, Org. Biomol. Chem., 2010, 8, 4394-4399. DOI: 10.1039/C0OB00219D
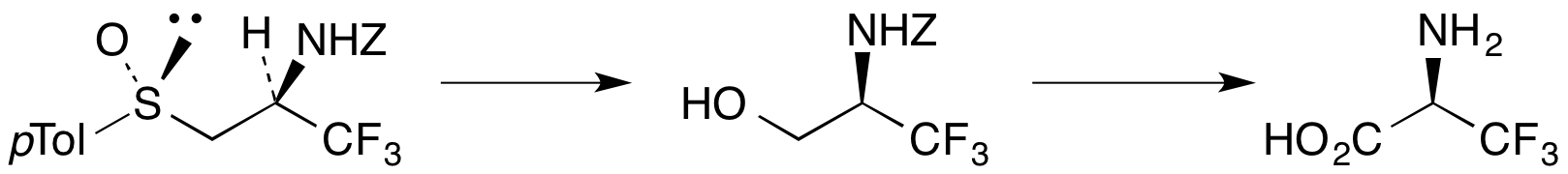
L. J. Drummond and A. Sutherland, Asymmetric Synthesis Of Allylic Secondary Alcohols: A New General Approach For The Preparation of α-Amino Acids, Tetrahedron, 2010, 66, 5349-5356. DOI: 10.1016/j.tet.2010.05.066
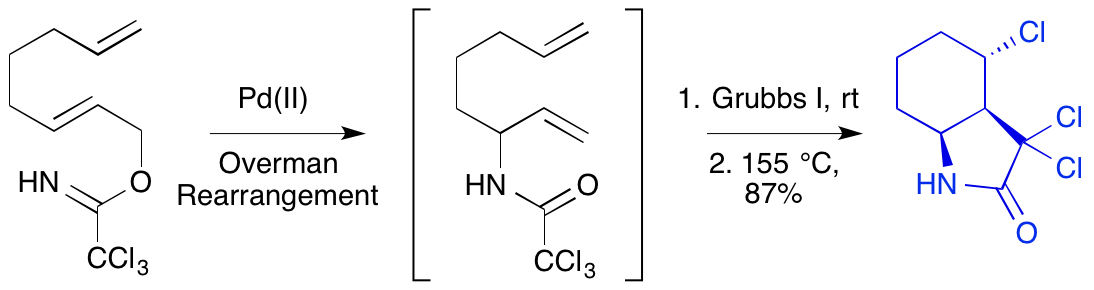
F. I. McGonagle, L. Brown, A. Cooke and A. Sutherland, A Three-Step Tandem Process For The Synthesis Of Bicyclic γ-Lactams, Org. Biomol. Chem., 2010, 8, 3418-3425. DOI: 10.1039/C004695G
 |
 |
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2010, 27, 149-152, 477-480, 805-808, 1110-1113, 1381-1385, 1733-1736.
2009
L. S. Fowler, D. Ellis and A. Sutherland, Synthesis Of Fluorescent Enone Derived α-Amino Acids, Org. Biomol. Chem., 2009, 7, 4309-4316. DOI: 10.1039/B912782H
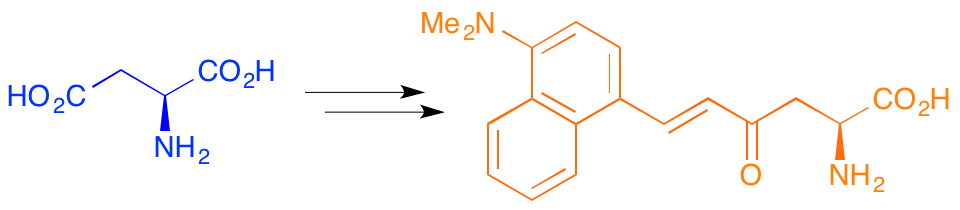
C. M. Reid and A. Sutherland, Synthesis Of Isotopically Labeled α-Amino Acids, Chapter 11 In Volume 1 Of Amino Acids, Peptides And Proteins In Organic Chemistry, Ed., A. B. Hughes, Wiley-VCH, Weinheim, 2009.
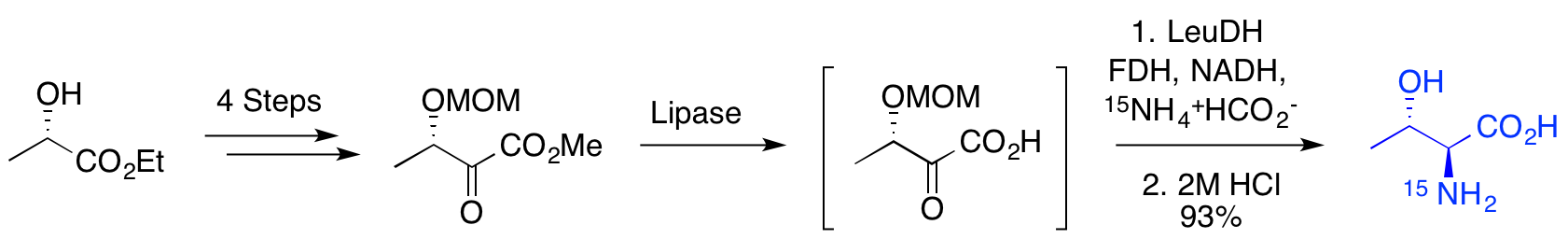
N. K. Jobson, A. R. Crawford, D. Dewar, S. L. Pimlott and A. Sutherland, Design And Synthesis Of (2R,3S)-Iodoreboxetine Analogues For SPECT Imaging Of The Noradrenaline Transporter, Bioorg. Med. Chem. Lett., 2009, 19, 4996-4998. DOI: 10.1016/j.bmcl.2009.07.064
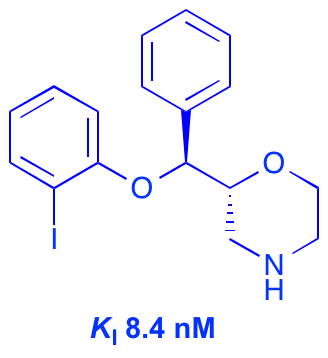
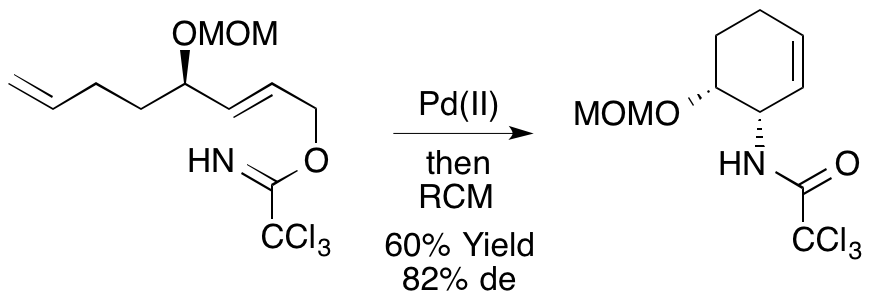
A. M. Zaed, M. D. Swift and A. Sutherland, A Stereoselective Synthesis Of (+)-Physoperuvine Using A Tandem Aza-Claisen Rearrangement And Ring Closing Metathesis Reaction, Org. Biomol. Chem., 2009, 7, 2678-2680. DOI: 10.1039/B907341H
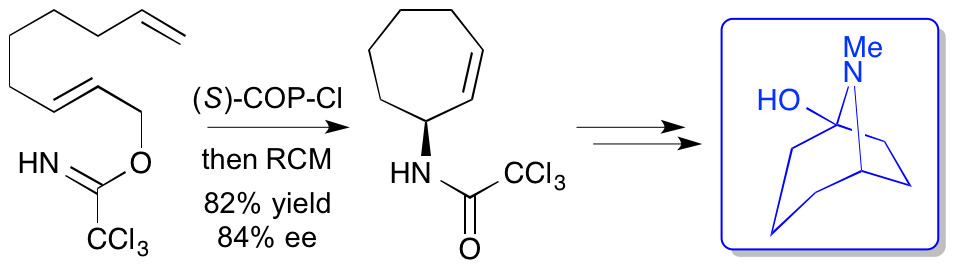
|
 |
M. D. Swift, A. Donaldson and A. Sutherland, Tandem Aza-Claisen Rearrangement And Ring Closing Metathesis Reactions: The Stereoselective Synthesis Of Functionalised Carbocyclic Amides, Tetrahedron Lett., 2009, 50, 3241-3244. Invited contribution to a special 50th anniversary issue. DOI: 10.1016/j.tetlet.2009.02.032
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2009, 26, 151-154, 461-464, 725-728, 973-976, 1229-1233, 1517-1520.
2008
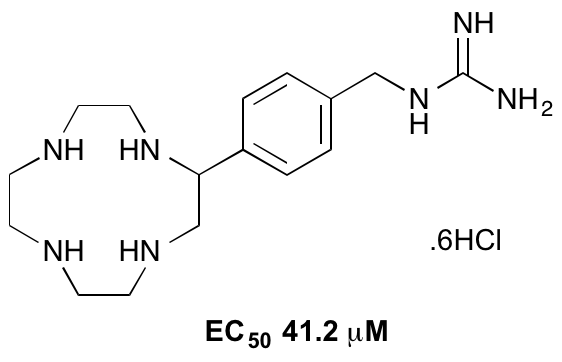
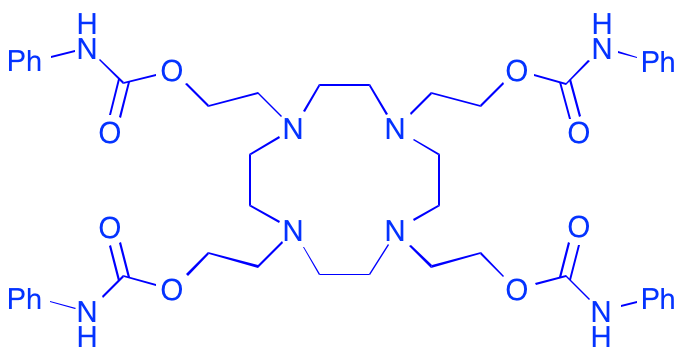
C. M. Reid, C. Ebikeme, M. P. Barrett, E.-M. Patzewitz, S. Muller, D. J. Robins and A. Sutherland, Synthesis Of Novel Benzamidine- And Guanidine-Derived Polyazamacrocycles: Selective Anti-Protozoal Activity For Human African Trypanosomiasis, Bioorg. Med. Chem. Lett., 2008, 18, 5399-5401. DOI: 10.1016/j.bmcl.2008.09.047
N. K. Jobson, A. R. Crawford, D. Dewar, S. L. Pimlott and A. Sutherland, New Iodoreboxetine Analogues For SPECT Imaging Of The Noradrenaline Transporter, Bioorg. Med. Chem. Lett., 2008, 18, 4940-4943. DOI: 10.1016/j.bmcl.2008.08.041
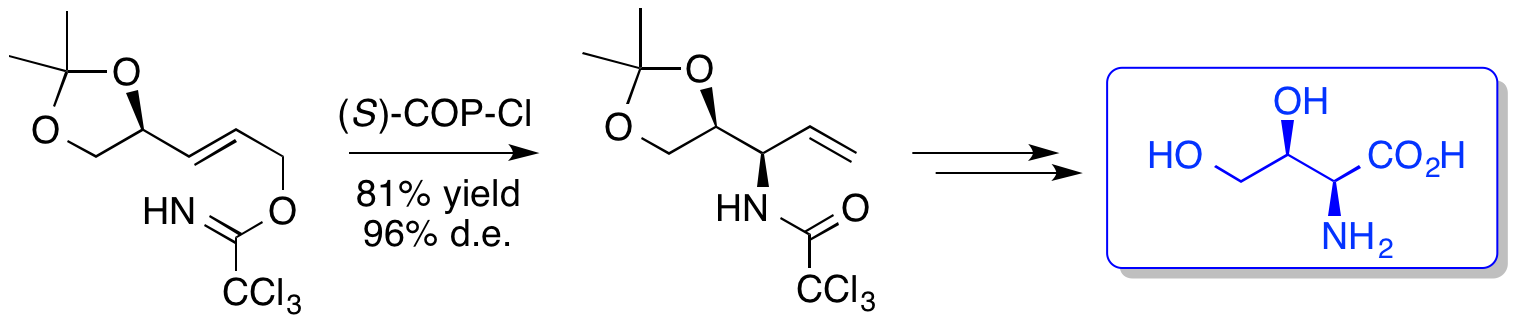
M. D. Swift and A. Sutherland, Studies On The aza-Claisen Rearrangement of 4,5-Dihydroxylated Allylic Trichloroacetimidates: The Stereoselective Synthesis Of (2R,3S)- And (2S,3S)-2-Amino-3,4-Dihydroxybutyric Acids, Tetrahedron, 2008, 64, 9521-9527. DOI: 10.1016/j.tet.2008.07.062
N. K. Jobson, R. Spike, A. R. Crawford, D. Dewar, S. L. Pimlott and A. Sutherland, Stereoselective Synthesis of (2S,3R)- and (2R,3S)-iodoreboxetine; Potential SPECT Imaging Agents For The Noradrenaline Transporter, Org. Biomol. Chem., 2008, 6, 2369-2376. DOI: 10.1039/B802819B
S. L. Pimlott, L. Stevenson, D. J. Wyper and A. Sutherland, Rapid And Efficient Radiosynthesis of [123I]-I-PK11195, A SPECT Tracer For The Peripheral Benzodiazepine Receptors, Nucl. Med. Biol., 2008, 35, 537-542. DOI: 10.1016/j.nucmedbio.2008.02.013
C. M. Reid, C. Ebikeme, M. P. Barrett, E.-M. Patzewitz, S. Muller, D. J. Robins and A. Sutherland, Synthesis And Anti-protozoal Activity Of C2-Substituted Polyazamacrocycles, Bioorg. Med. Chem. Lett., 2008, 18, 2455-2458. DOI: 10.1016/j.bmcl.2008.02.037
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2008, 25, 11-14, 216-219, 443-446, 647-650, 841-844, 997-1000.
2007
M. D. Swift and A. Sutherland, A Tandem Aza-Claisen Rearrangement And Ring Closing Metathesis Reaction For The Synthesis of Cyclic Allylic Trichloroacetamides, Org. Lett., 2007, 9, 5239-5242. DOI: 10.1021/ol702299c
K. N. Fanning and A. Sutherland, A Facile Synthesis of (S)-Gizzerosine, A Potent Agonist Of The Histamine H2-Receptor, Tetrahedron Lett., 2007, 48, 8479-8481. DOI: 10.1016/j.tetlet.2007.09.165
J. M. Wilson, F. Giordani, L. J. Farugia, M. P. Barrett, D. J. Robins and A. Sutherland, Synthesis, Characterisation and Anti-Protozoal Activity Of Carbamate-Derived Polyazamacrocycles, Org. Biomol. Chem., 2007, 5, 3651-3656. DOI: 10.1039/B710487A
 |
 |
L. Stevenson, S. L. Pimlott and A. Sutherland, A Novel Approach For The Synthesis Of The Peripheral Benzodiazepine Receptor Ligand, PK11195, Tetrahedron Lett., 2007, 48, 7137-7139. DOI: 10.1016/j.tetlet.2007.07.203
R. Bischoff, D. J. Hamilton, N. K. Jobson and A. Sutherland, New Approaches For The Synthesis Of Isotopically Labelled Guanidine-Derived Amino Acids and Noradrenaline Reuptake Inhibitors, J. Labelled Compds. Radiopharm., 2007, 50, 323-326. DOI: 10.1002/jlcr.1242
M. D. Swift and A. Sutherland, A Stereoselective Synthesis of (2R,3S)-2-Amino-3,4-Dihydroxybutyric Acid Using An Ether Directed aza-Claisen Rearrangement, Tetrahedron Lett., 2007, 48, 3771-3773. DOI: 10.1016/j.tetlet.2007.03.161
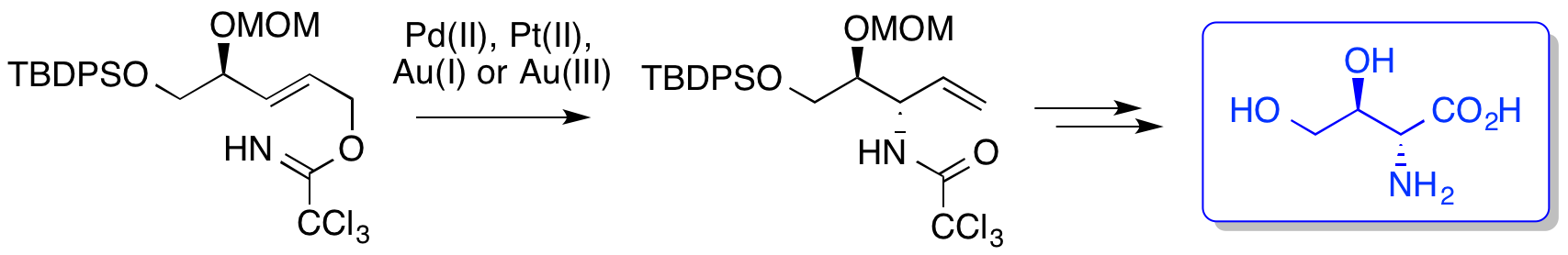
A. G. Jamieson and A. Sutherland, Ether-Directed, Stereoselective Aza-Claisen Rearrangements: Synthesis Of The Piperidine Alkaloid, α-Conhydrine, Org. Lett., 2007, 9, 1609-1611. DOI: 10.1021/ol070424z
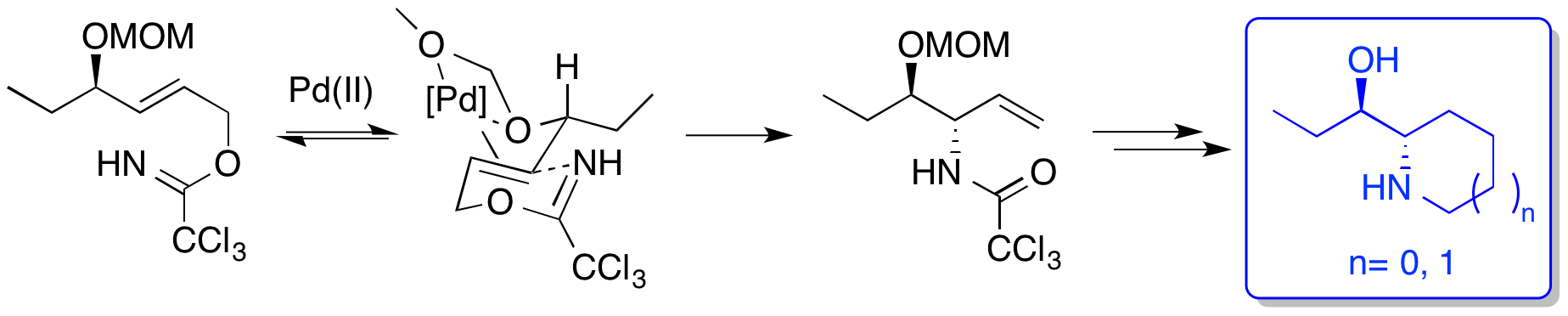
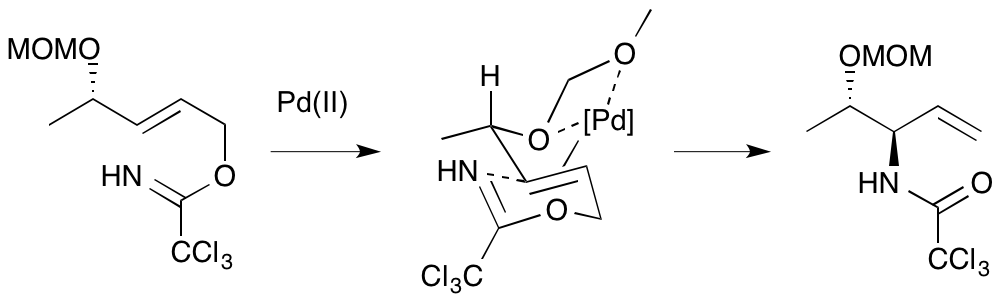
A. G. Jamieson and A. Sutherland, Ether-Directed Palladium(II)-Catalysed Aza-Claisen Rearrangements: Studies On The Origin Of The Directing Effect, Tetrahedron, 2007, 63, 2123-2131. DOI: 10.1016/j.tet.2006.12.067
J. M. Wilson, G. Henderson, F. Black, A. Sutherland, R. L. Ludwig, K. H. Vousden and D. J. Robins, Synthesis of 5-Deazaflavin Derivatives And Their Activation Of p53 in Cells, Bioorg. Med. Chem., 2007, 15, 77-86. DOI: 10.1039/b710487a
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2007, 24, 14-17, 263-266, 500-503, 655-658, 927-930, 1207-1210.
2006
M. D. Swift and A. Sutherland, Stereocontrol Of Palladium(II)-Catalysed aza-Claisen Rearrangements Using A Combination Of 1,3-Allylic Strain And A Solvent Mediated Directing Effect, Org. Biomol. Chem., 2006, 4, 3889-3891. DOI: 10.1039/B613271E
A. G. Jamieson and A. Sutherland, Scope And Limitations Of Ether-Directed, Metal-Catalysed aza-Claisen Rearrangements; Improved Stereoselectivity Using Non-coordinating Solvents, Org. Biomol. Chem., 2006, 4, 2932-2937. DOI: 10.1039/B607014K
K. N. Fanning, A. G. Jamieson and A. Sutherland, Palladium(II)-Catalysed Rearrangement Reactions, Curr. Org. Chem., 2006, 10, 1007-1020.
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2006, 23, 11-14, 143-146, 343-346, 513-516, 669-672, 841-844.
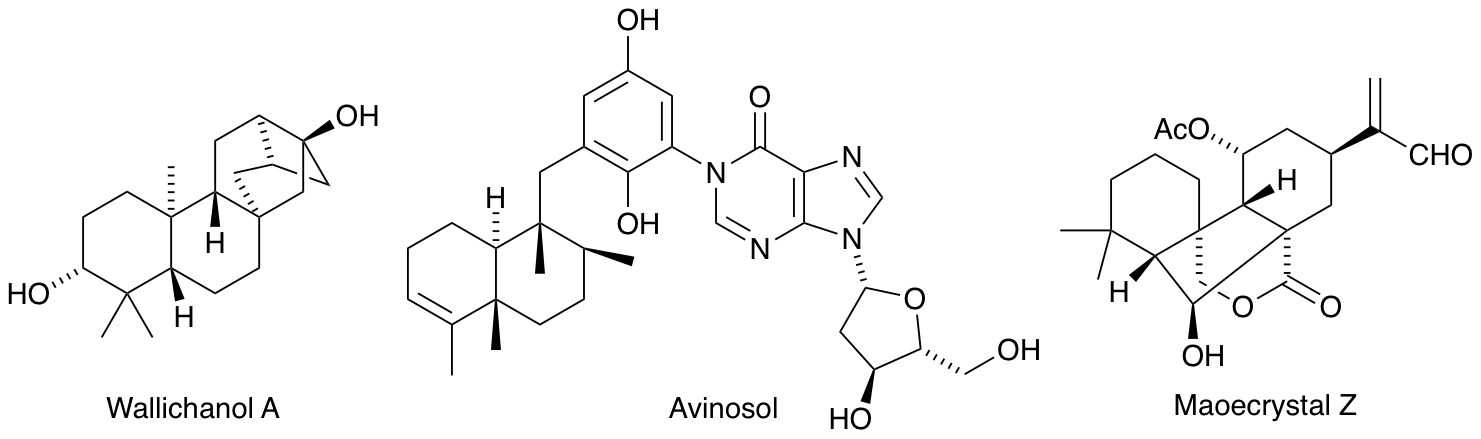

R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2005, 22, 11-14, 141-143, 320-323, 436-438, 559-562, 668-671.
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2004, 21, H1-H4, H5-H8, H9-H12, H13-H15, H17-H21, H23-H26.
B. Pillai, M. Cherney, C. M. Diaper, A. Sutherland, J. S. Blanchard, J. C. Vederas and M. N. G. James, Dynamics of Catalysis Revealed From Crystal Structures of Mutants of Diaminopimelate Epimerase, Biochem. Biophys. Res. Commun., 2007, 363, 547-553. DOI: 10.1016/j.bbrc.2007.09.012

2006
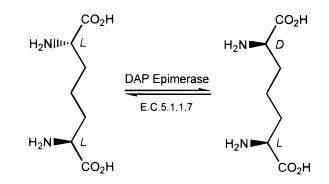
B. Pillai, M. M. Cherney, C. M. Diaper, A. Sutherland, J. S. Blanchard, J. C. Vederas and M. N. G. James, Structural Insights Into Stereochemical Inversion By Diaminopimelate Epimerase: An Antibacterial Drug Target, Proc. Natl. Acad. Sci. USA, 2006, 103, 8668-8673. DOI: 10.1073/pnas.0602537103
C. M. Diaper, A. Sutherland, B. Pillai, M. N. G. James, P. Semchuk, J. S. Blanchard and J. C. Vederas, The Stereoselective Synthesis Of Aziridine Analogues Of Diaminopimelic Acid (DAP) And Their Interaction With DAP Epimerase, Org. Biomol. Chem., 2005, 3, 4402-4411. DOI: 10.1039/B513409A
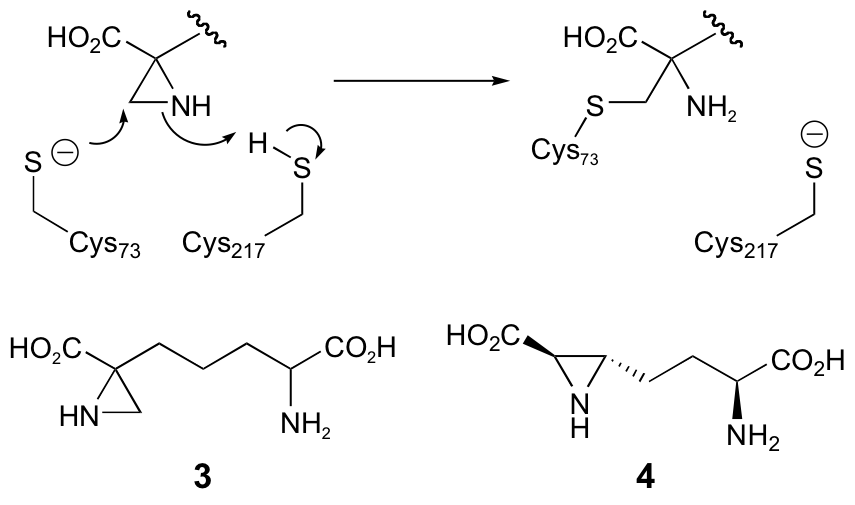 |
 |
2003
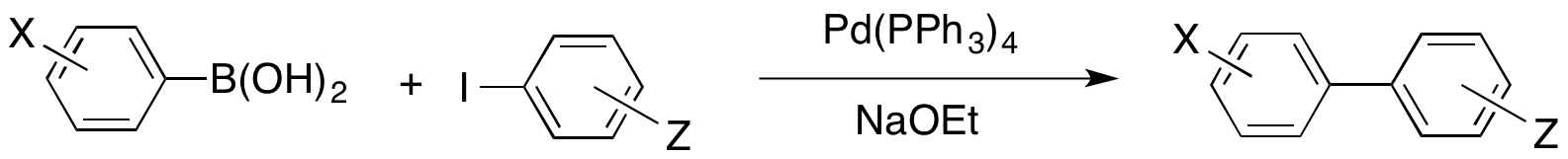
G. Karig, J. M. Large, C. G. V. Sharples, A. Sutherland, T. Gallagher and S. Wonnacott, Synthesis and Nicotinic Binding Of Novel Phenyl Derivatives Of UB-165. Identifying Factors Associated with α-7 Selectivity, Bioorg. Med. Chem. Lett., 2003, 13, 2825-2828. DOI: 10.1016/S0960-894X(03)00594-8

A. Sutherland and T. Gallagher, The Synthesis And Reactivity Of Halopyridylboronic Acids, Reactivity, 2003, 4-7.
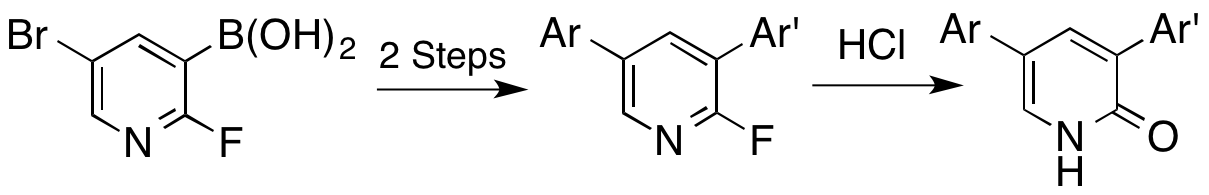
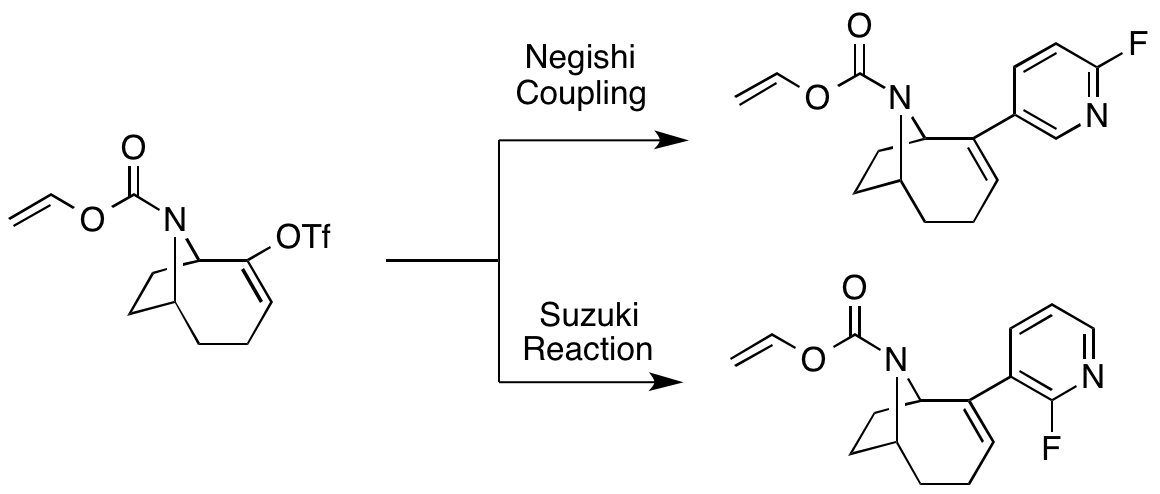
A. Sutherland and T. Gallagher, Versatile Synthesis Of 3,5-Disubstituted 2-Fluoropyridines And 2-Pyridones, J. Org. Chem., 2003, 68, 3352-3355. DOI: 10.1021/jo026864f
A. Sutherland, T. Gallagher, C. G. V. Sharples and S. Wonnacott; The Synthesis Of Two Fluoro Analogues Of The Nicotinic Acetylcholine Receptor Agonist UB-165, J. Org. Chem., 2003, 68, 2475-2478. DOI: 10.1021/jo026698b
R. A. Hill and A. Sutherland, Hot Off The Press, Nat. Prod. Rep., 2003, 20, xlv-xlviii.
2002
A. Sutherland and J. C. Vederas, Conjugate Addition Of Radicals Generated From Diacyloxyiodobenzenes to Dehydroamino Acid Derivatives; A Synthesis of Diaminopimelic Acid Analogues, Chem. Commun., 2002, 224-225. DOI: 10.1039/B109343F
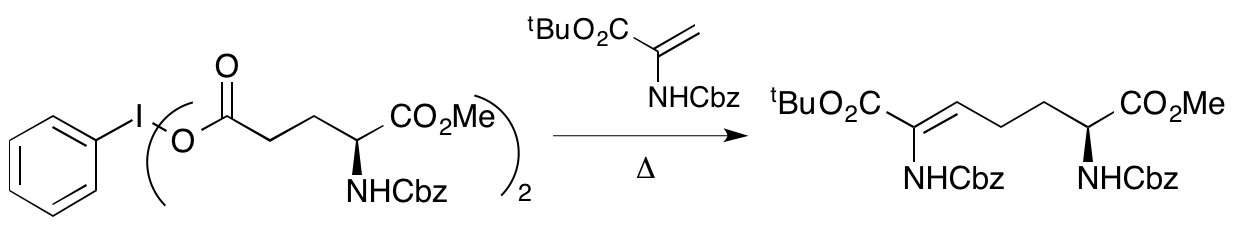
2001
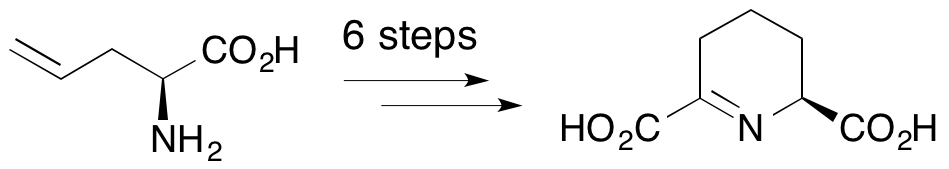
J. F. Caplan, A. Sutherland and J. C. Vederas, The First Stereospecific Synthesis of L-Tetrahydrodipicolinic Acid; A Key Intermediate Of Diaminopimelate Metabolism, J. Chem. Soc., Perkin Trans. 1, 2001, 2217-2220. DOI: 10.1039/B105091P
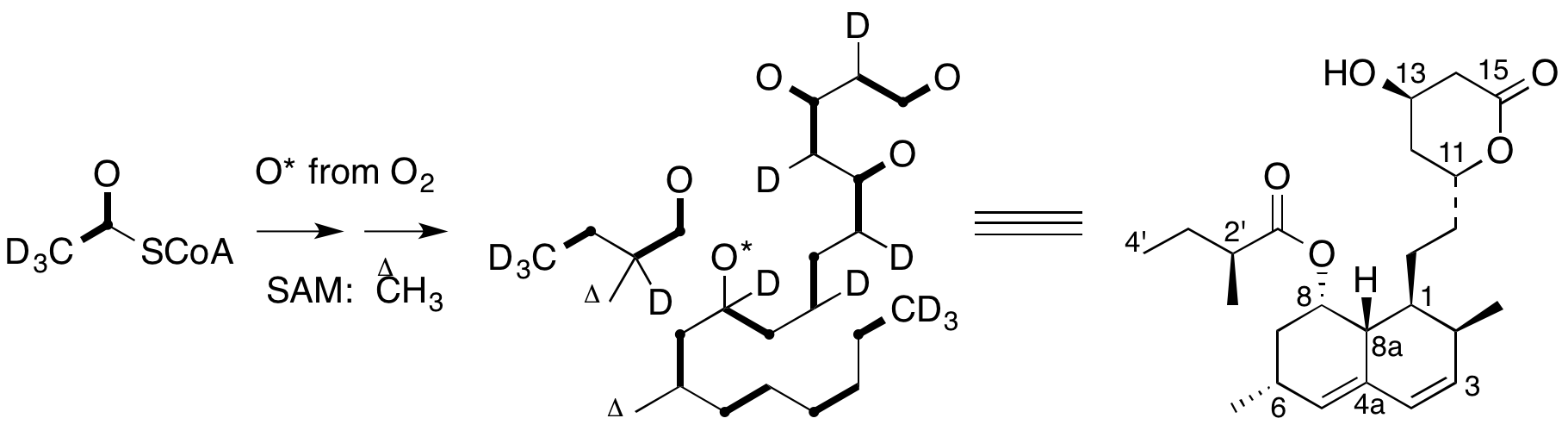
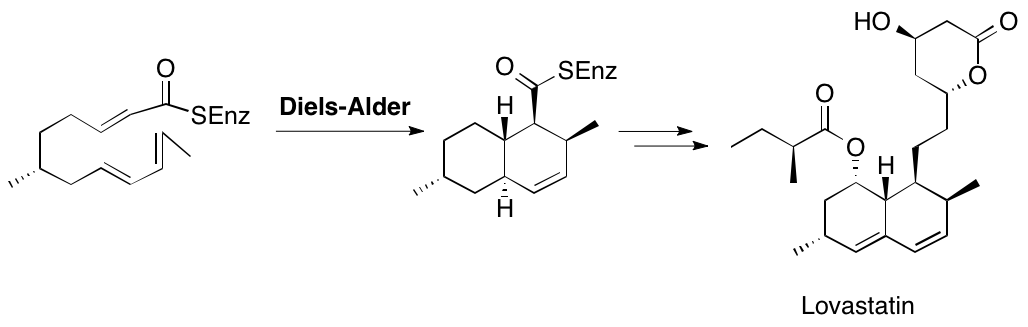
A. Sutherland, K. Auclair and J. C. Vederas, Recent Advances In The Biosynthetic Studies Of Lovastatin, Current Opinion in Drug Discovery and Development, 2001, 4, 229-236.
2000
K. Auclair, A. Sutherland, J. Kennedy, D. J. Witter, J. P. Van den Heever, C. R. Hutchinson and J. C. Vederas, Lovastatin Nonaketide Synthase Catalyses An Intramolecular Diels-Alder Reaction Of A Substrate Analogue, J. Am. Chem. Soc., 2000, 122, 11519-11520. DOI: 10.1021/ja003216+
A. Sutherland and C. L. Willis, Synthesis Of Fluorinated Amino Acids, Nat. Prod. Rep., 2000, 17, 621-631. DOI: 10.1039/A707503K
M. Cirilli, G. Scapin, A. Sutherland, J. C. Vederas and J. S. Blanchard, The Three-Dimensional Structure Of The Ternary Complex Of Corneybacterium glutamicum Diaminopimelate Dehydrogenase-NADPH-L-2-Amino-6-Methylene-Pimelate, Protein Science, 2000, 9, 2034-2037. DOI: 10.1110/ps.9.10.2034
J. R. Harding, R. A. Hughes, N. M. Kelly, A. Sutherland and C. L. Willis, Syntheses of Isotopically Labelled L-α-Amino Acids With An Asymmetric Centre at C-3, J. Chem. Soc., Perkin Trans. 1, 2000, 3406-3416. DOI: 10.1039/B005172L
C. W. Koo, A. Sutherland, J. C. Vederas and J. S. Blanchard, Identification of Active Site Cysteine Residues That Function As General Bases: Diaminopimelate Epimerase, J. Am. Chem. Soc., 2000, 122, 6122-6123. DOI: 10.1021/ja001193t
R. J. Cox, A. Sutherland and J. C. Vederas, Bacterial Diaminopimelate Metabolism As A Target For Antibiotic Design, Bioorg. Med. Chem., 2000, 8, 843-871. DOI: 10.1016/S0968-0896(00)00044-4
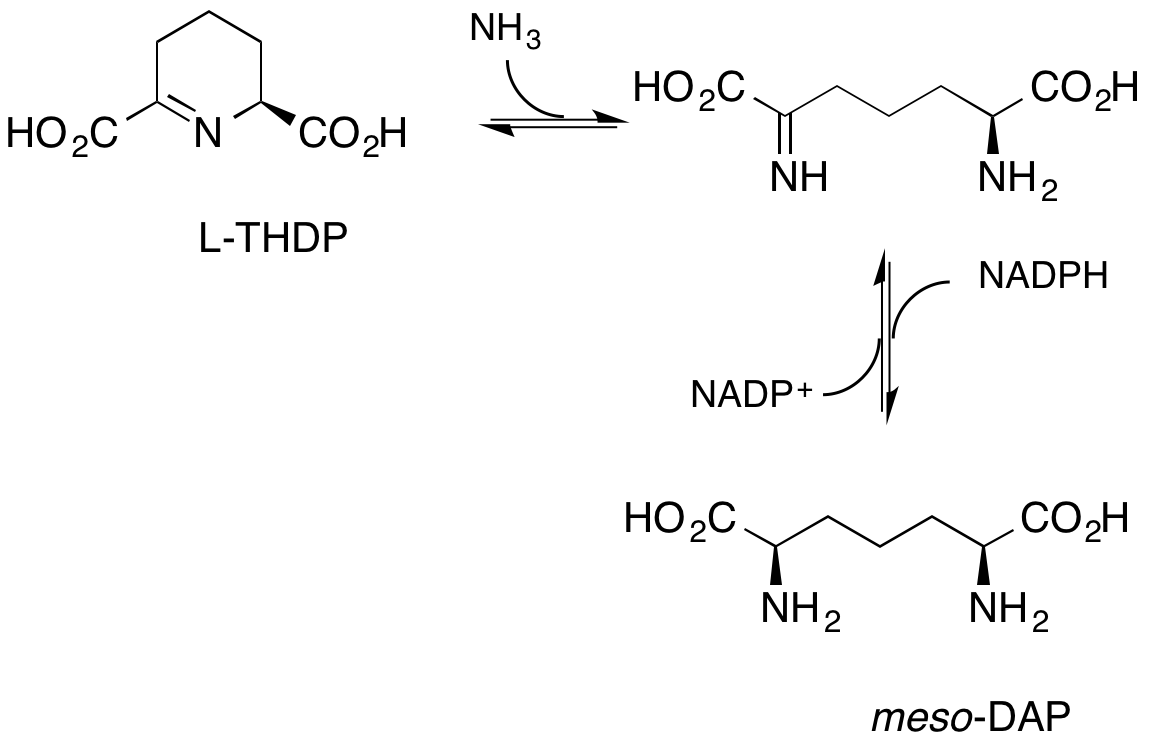
G. Bhalay, S. Clough, L. McLaren, A. Sutherland and C. L. Willis, Synthesis and Enzyme-Catalysed Reductions Of 2-Oxo Acids With Oxygen Containing Side-Chains, J. Chem. Soc., Perkin Trans. 1, 2000, 901-910. DOI: 10.1039/A909677I
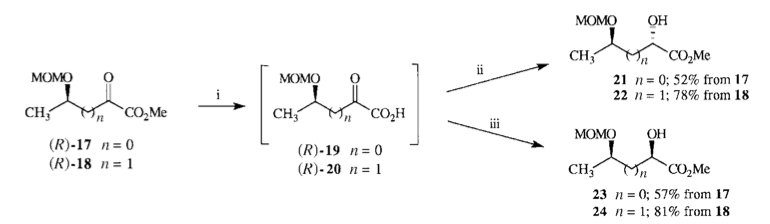
M. D. Fletcher, J. R. Harding, R. A. Hughes, N. M. Kelly, H. Schmalz, A. Sutherland and C. L. Willis, Three Approaches To The Synthesis Of L-Leucine Selectively Labelled With Carbon-13 Or Deuterium In Either Diastereotopic Methyl Group, J. Chem. Soc., Perkin Trans. 1, 2000, 43-52. DOI: 10.1039/A907598D
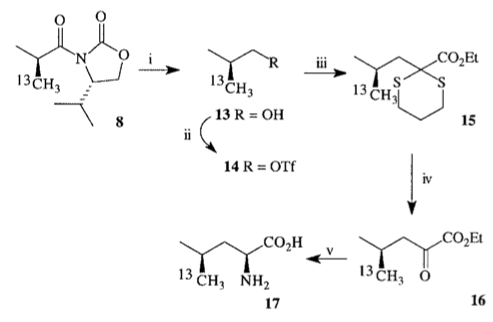
1999
A. Sutherland and J. C. Vederas, The First Isolation Of An Alkoxy-N,N-Dialkylaminodifluorosulfane From The Reaction Of An Alcohol And DAST: An Efficient Synthesis Of (2S,3R,6S)-3-Fluoro-2,6-Diaminopimelic Acid, Chem. Commun., 1999, 1739-1740. DOI: 10.1039/A904821I
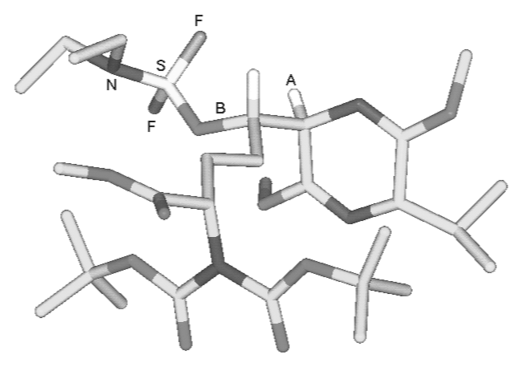
A. Sutherland and C. L. Willis, Synthesis Of Probes For The Active Site Of Leucine Dehydrogenase, Bioorg. Med. Chem. Lett., 1999, 9, 1941-1944. DOI: 10.1016/S0960-894X(99)00297-8
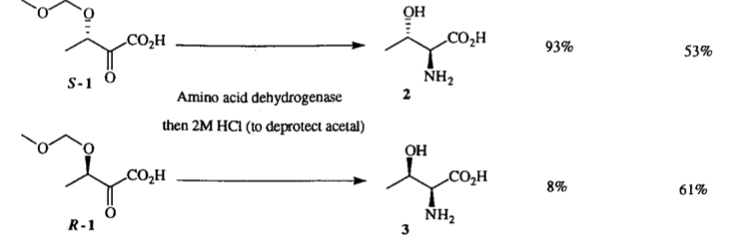
A. Sutherland, J. F. Caplan and J. C. Vederas, Unsaturated α-Aminopimelic Acids As Potent Inhibitors Of meso-Diaminopimelic Acid (DAP) D-Dehydrogenase, Chem. Commun., 1999, 555-556. DOI: 10.1039/A900297I
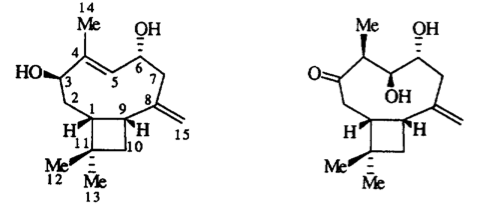
M. Frank, E. Kingston, J. C. Jeffery, M. O. Moss, M. Murray, T. J. Simpson and A. Sutherland, Walleminol and Walleminone, Novel Caryophyllenes From The Toxigenic Fungus Wallemia sebi, Tetrahedron Lett., 1999, 40, 133-136. DOI: 10.1016/S0040-4039(98)80039-7
1996-1998
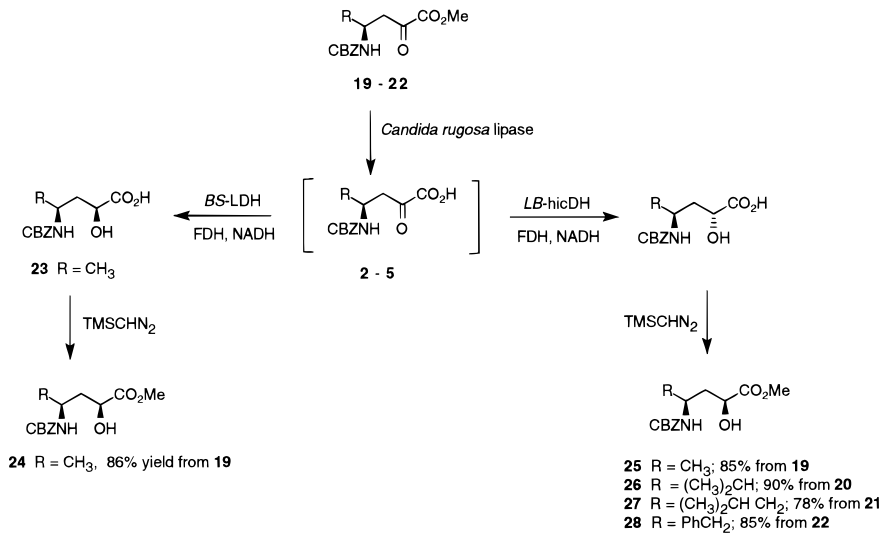
A. Sutherland and C. L. Willis, Chemoenzymatic Synthesis Of 4-Amino-2-Hydroxy Acids: A Comparison Of Mutant And Wild-Type Oxidoreductases, J. Org. Chem., 1998, 63, 7764-7769. DOI: 10.1021/jo980821a
M. D. Fletcher, N. M. Kelly, A. Sutherland and C. L. Willis, “EnantioselectiveSynthesis Of L-α-Amino Acids Incorporating Stable Isotopic Labels,” In “Synthesis And Applications Of Isotopically Labelled Compounds 1997,” eds. J. R. Heys and D. G. Melillo, Wiley, 1998, 161-164.
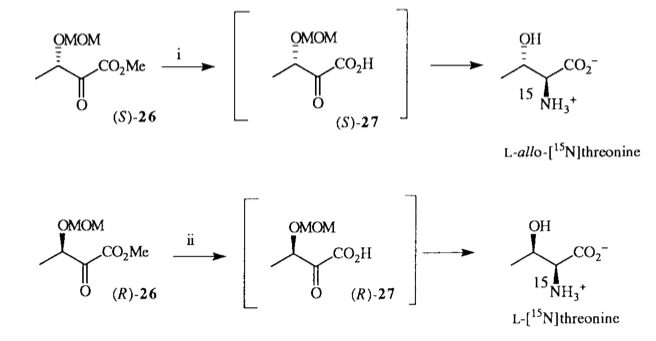
A. Sutherland and C. L. Willis, Synthesis Of α-Amino-β-Hydroxy Acids, [15N]-L-alloThreonine And [15N]-L-Threonine, Tetrahedron Lett., 1997, 38, 1837-1840. DOI: 10.1016/S0040-4039(97)00164-0
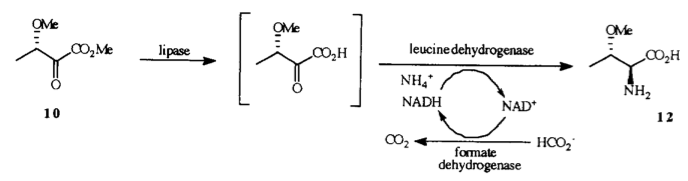
N. M. Kelly, A. Sutherland and C. L. Willis, Syntheses Of Amino Acids Incorporating Stable Isotopes, Nat. Prod. Rep., 1997, 14, 205-220. DOI: 10.1039/NP9971400205
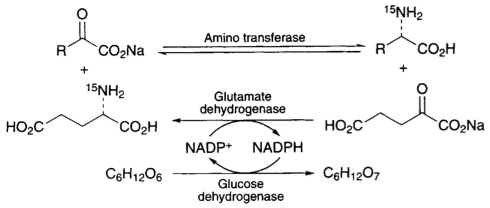
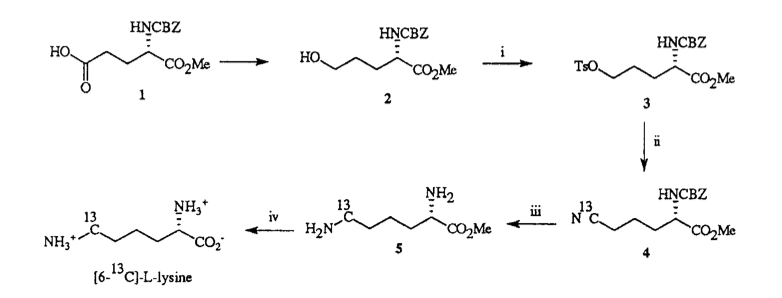
A. Sutherland and C. L. Willis, Synthesis Of [6-13C]-L-Lysine, J. Labelled Compds. Radiopharm., 1996, 38, 95-102. DOI: 10.1002/(SICI)1099-1344(199601)38:1<95::AID-JLCR816>3.0.CO;2-Q