Obtaining accurate charge densities in compounds with elements heavier than Z = 35 (Br) is one of the current challenges in this area. It is usually considered necessary to utilise short wavelength synchrotron X-radiation to minimise the effects of absorption and extinction.
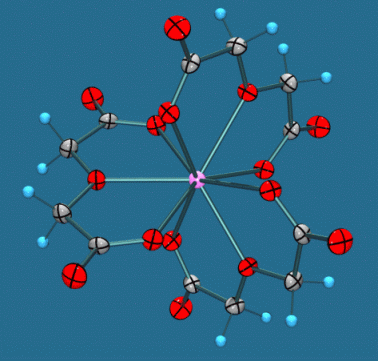
The lanthanide complexes of 2,2'-oxydiacetate Na5[M(C4H4O5)3] (BF4)2.6H2O (M=Nd, Sm, Eu, Gd) are isomorphous and isostructural, and contain 9-coordinate lanthanide ions (Fig 1). The racemic complexes spontaneously resolve on crystallisation in the trigonal space group R32 into the lambda and delta isomers, and their excellent large and well formed crystals have been shown to exhibit X-ray CD spectra (Peacock, Stewart et al).
Due to the unusually high crystallinity of these materials, we have obtained excellent quality data sets for the Gd and Eu complexes from a laboratory diffractometer (Nonius KappaCCD, 100K). In a recent article (J. Phys. Chem. Solids (2004) 65 1927-1933), Lecomte et al reported serious difficulties in modelling the charge density for a gadolinium complex, which was attributed to inadequate scattering factors for Gd. In contrast, we find no such difficulty using the new XD scattering factors obtained from STO atomic relativistic wavefunctions obtained at PBE/QZ4P level of theory (Volkov A.; Macchi, P. unpublished work).
|
|
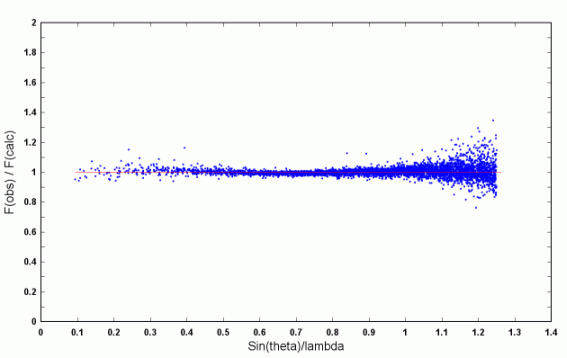
| Figure 1. The structure of the anionic complex [Gd(C4H4O5)3]3-. | Figure 2. Scatterplot of scale factors for individual reflections. |
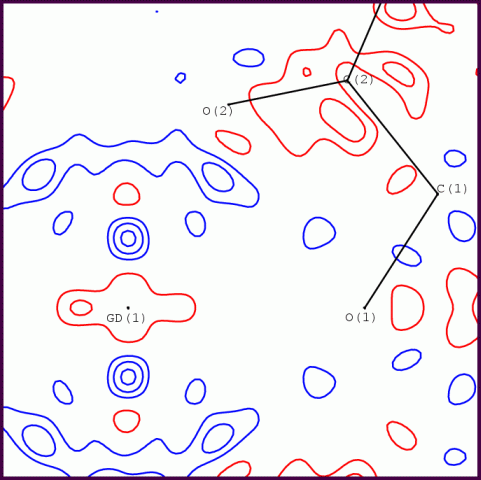
A multipole refinement using XD2006 converged to R(F) = 0.65% with GOF = 1.26, for 5264 data with sin(theta)/lambda <= 1.08. All data have I > 3sig(I). The residuals in a Fourier map (max sin(theta)/lambda = 1) range from -0.31 to +0.26 eA-3, which is quite acceptable for such a heavy atom as Gd (Z=64). It was found necessary to adopt anharmonic thermal motion for the Gd and Na atoms to model the sharp residual features around these atoms. In order to cope with the substantially differing radial properties of the 6s/5d and 4f valence electrons, the Gd atom was modelled by two coincident pseudoatoms - the corresponding entry in the SCAT table is shown below.