Research in the Sutherland group is
focused on a number of key areas. Our main programme of research
centres on molecular imaging of disease. We have a number of
projects that develop novel PET and SPECT tracers for the
radionuclide imaging of a range of neurological diseases as well
as cancer. We are also investigating amino acid based fluorescent
probes for cell imaging. Supporting these projects is a synthetic
chemistry programme, which seeks to develop new transition metal
catalysed transformations and one-pot multi-reaction processes for
the rapid preparation of polycyclic drug-like scaffolds and
natural products.
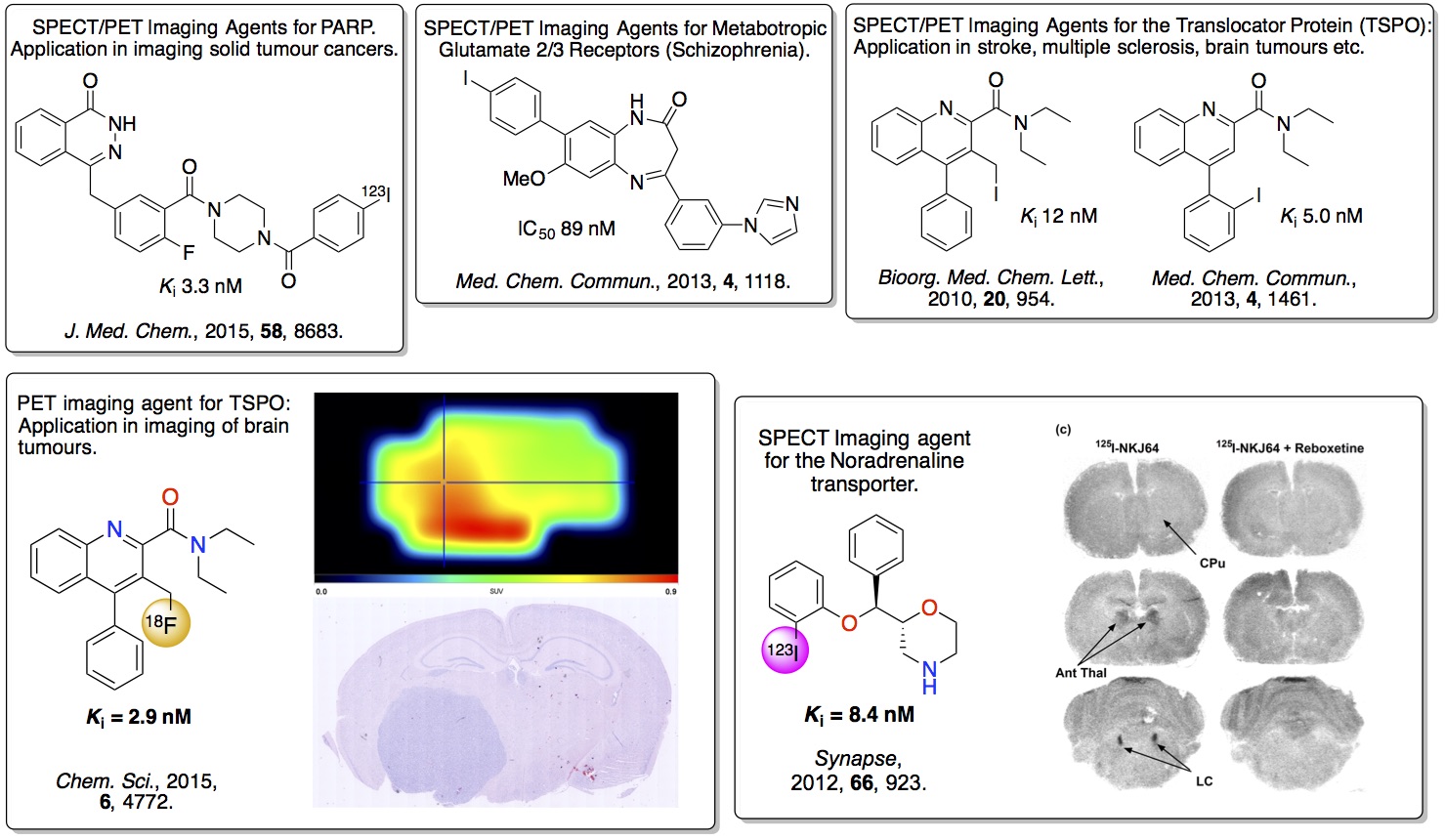
1. Novel PET and SPECT Tracers
for the Radionuclide Imaging of Disease: Many diseases
are poorly diagnosed and treated due to a lack of understanding at
the molecular level. One approach in gaining a better insight into
disease mechanisms, is the design of non-toxic, molecular imaging
agents that can bind with high affinity and high selectivity to a
targeted biological receptor. The challenge is to generate
functionalised molecular tracers that can produce insightful
images of a specific disease. Our work has identified a number of
high affinity
agents that can bind with high
selectivity to neurological receptors involved in dementia
associated disease and cancer. In particular, a PET imaging agent
has been developed that binds to the translocator protein and can
be used to image brain tumours. In other work, a SPECT imaging
agent has been developed that can be used to image the
noradrenaline reuptake transporter in mice.
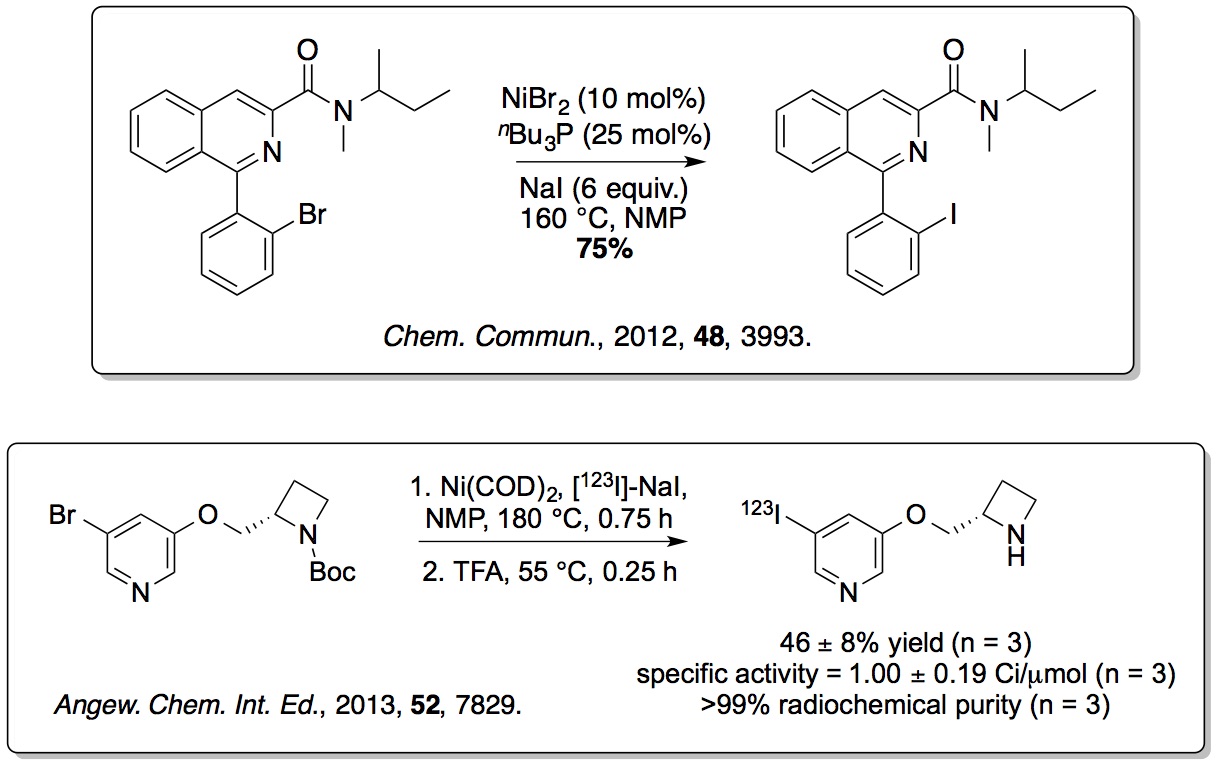
To overcome the limitations of using traditional highly toxic
(organotin) methods for generating radioiodinated SPECT imaging
agents, new non-toxic nickel-mediated halogen exchange reactions
have been developed. More recently, iron(III) and
silver(I)-catalysed methods for the mild iodination of arenes have
also been reported (
Org. Lett.,
2015,
17, 4782;
J. Org. Chem., 2016,
81, 772).
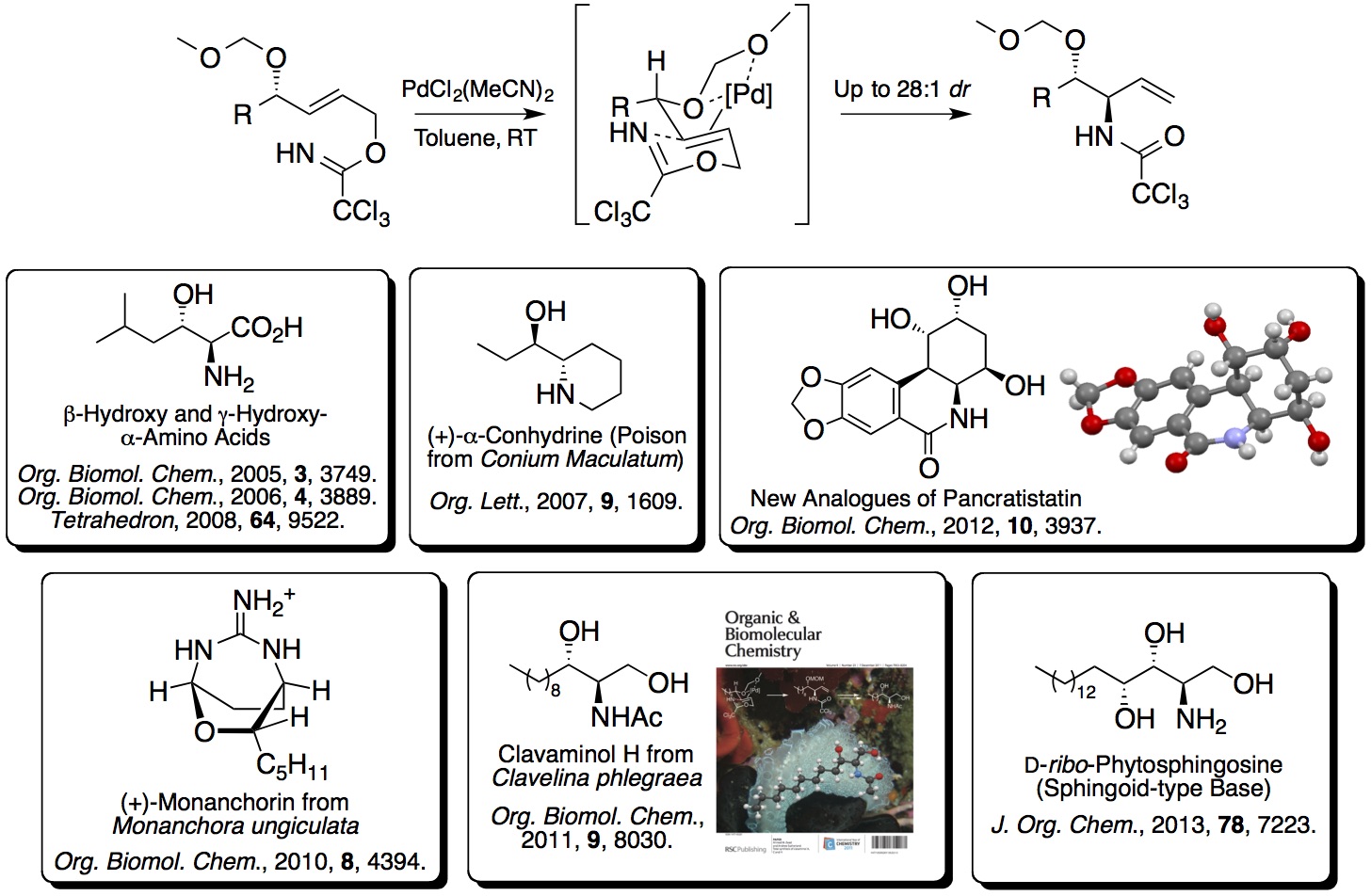
2. Directed Overman Rearrangements and One-Pot
Multi-reaction Processes for the Synthesis of Highly Functional
Building Blocks and Targets: An early project developed
in the group showed that methoxymethyl ethers were effective
directing groups for a diastereoselective palladium(II)-catalysed
Overman rearrangement. The resulting allylic
anti-vicinal amino alcohol
derivatives were excellent synthetic building blocks for a wide
range of drug-like compounds and natural products.
More recently, our interest in rearrangements, have led to the
design of new substrates that can be used in one-pot
multi-reaction processes for the rapid and highly efficient
synthesis of synthetic building blocks, polycyclic scaffolds and
natural products. In many of these processes, it has been shown
that a single transtion metal catalyst can be used to implement
different transformations.
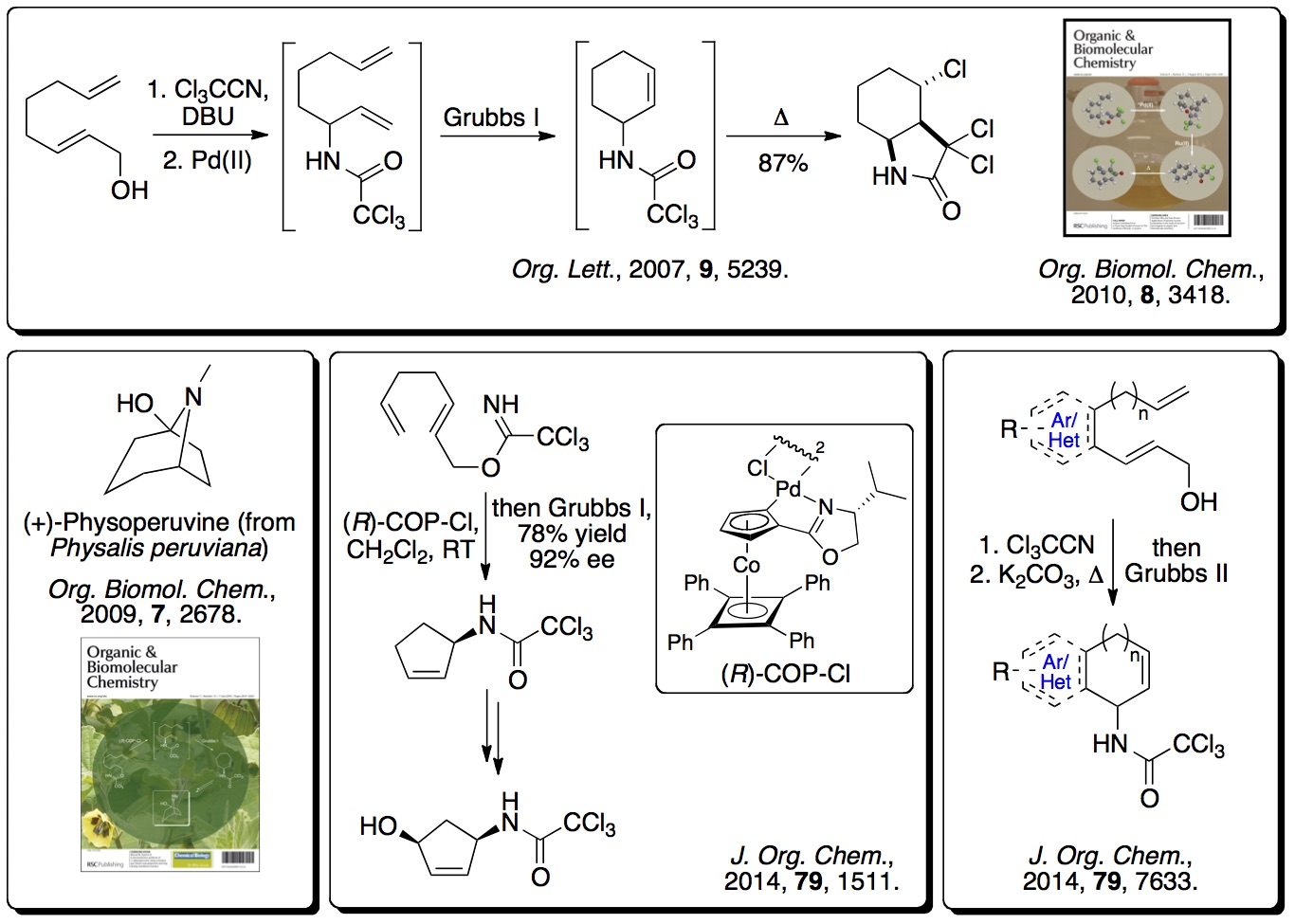
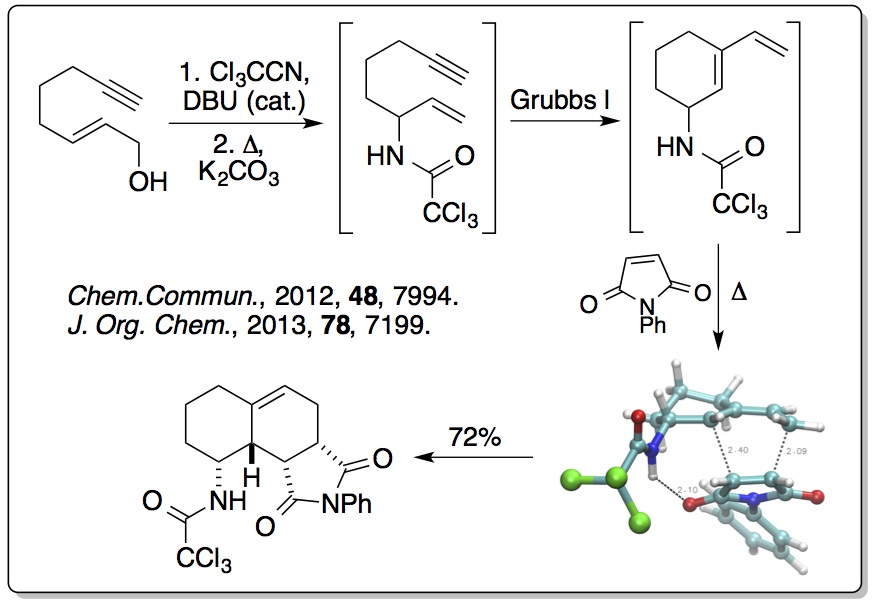
More recently, this concept has been extended to alkyne derived
allylic alcohols where one-pot processes have been used, involving
an Overman rearrangement, a ring closing enyne metathesis (RCEYM)
reaction and a hydrogen bonding directed Diels-Alder reaction,
generating polycyclic compounds with high diastereocontrol
(>20:1). This has been extended to include a one-pot process
involving ruthenium(II) tandem catalytic RCEYM and
cross-metathesis steps, generating highly substituted
amino-indanes and tetralins with up to five stereocentres (
Tetrahedron, 2014,
70, 7133). The RCEYM step has
also been fully investigated with disubstituted alkynes, allowing
a palladium catalyst to be used for the Overman rearrangement (
Org. Biomol. Chem., 2016,
14, 3284).
3. Use of Enone
Derived Amino Acids for the Synthesis of Piperidine Targets and
Fluorescent Imaging Agents: We have a general interest
in the novel and efficient synthesis of amino acids of particular
biological importance. For a number of years, we have been
exploring the chemical and biological applications of enone
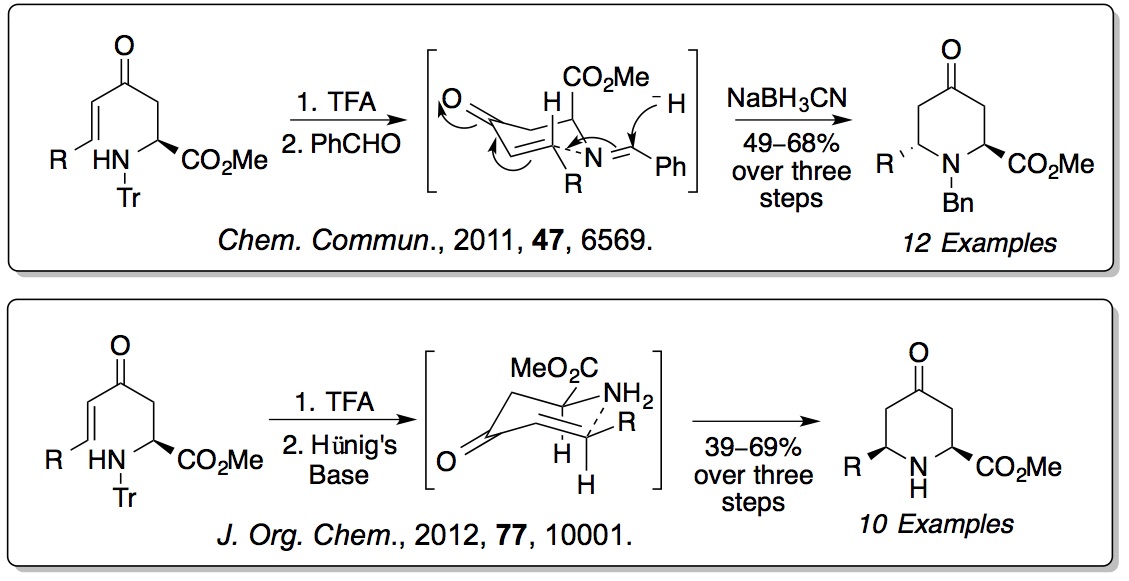
derived amino acids. We have shown that these compounds can
undergo 6-
endo-
trig cyclisations to form
pipecolic acids. The stereochemical outcome of these reactions can
be switched depending on the level of substitution on the amine.
Enone derived amino acids are also being explored as substrates
for heterocyclisations and as fluorescent probes for biological
imaging (
Org. Biomol. Chem.,
2009,
7, 4309;
Org. Biomol. Chem., 2015,
13, 4514).
Acknowledgements: We are grateful to the
following organisations for their financial support of our work.