The Symes group is interested in all aspects of electrochemistry and energy conversion in chemical systems. In the first instance, this means using electrical, photochemical and sonochemical inputs to drive chemical reactions that might not happen otherwise. A cornerstone of our approach is using renewable (or potentially renewable) energy sources to drive unfavourable or slow chemical reactions to deliver fuels and other high-value commodities.

Electrochemical water splitting and Decoupled Electrolysis
Splitting water to give hydrogen and oxygen is one route by which intermittent, renewable electricity (e.g. from a solar panel or wind turbine) could be stored as fuel. As such, it is vitally important that we develop new electrocatalysts for both the water oxidation and proton reduction reactions that do not rely on precious metals (the current industry standard).
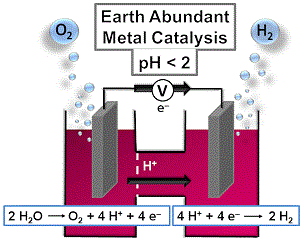
Previously, We showed that first row transition metals can catalyse electrochemical water splitting at pH < 2 . This was the first time that sustained oxygen and hydrogen evolution from water have been demonstrated at such low pH using only first row transition metals.
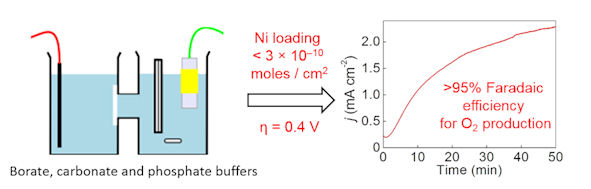
We have also shown that nanomolar concentrations of nickel in solution can catalyse electrochemical water splitting at neutral and basic pH. This is extremely important when trying to identify new water oxidation catalysts: new catalysts must henceforth demonstrate that adventitious nickel contamination is not the cause of any water oxidation activity that is observed. Our paper on this was published in JACS in 2015.
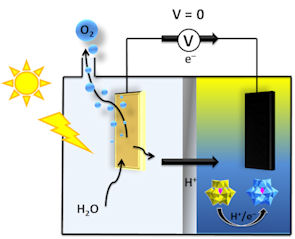
We are also very interested in "Decoupled Electrolysis", whereby the oxygen and hydrogen evolving steps take place at different times, and at rates that can be different from each other. This can bring advantages for catalyst loadings, product separation and purity, as well as allowing light-driven solar-to-hydrogen devices to be realised. The reduced mediators that enable decoupled electrolysis can also be used for chemical synthesis and in redox flow batteries. See our recent review of the field here.

New routes to nitrogen fixation
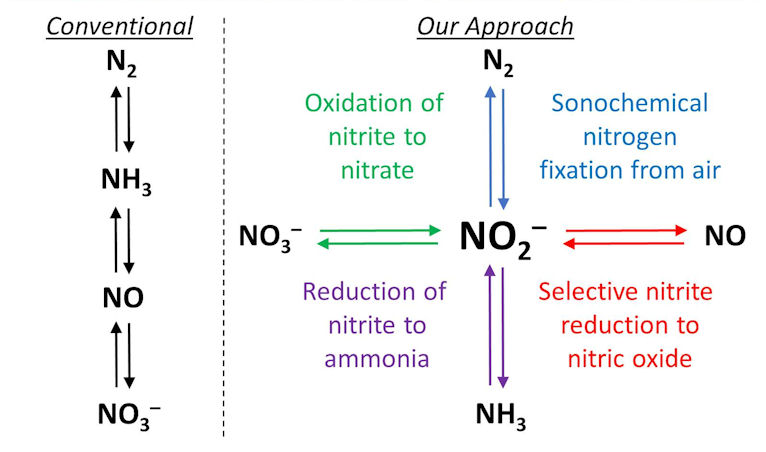
We are also interested in exploring alternative routes to nitrogen fixation, such as electrochemical ammonia production (check our our review of this field here) and the oxidative fixation of nitrogen by sonochemical methods. This latter approach might allow much milder conditions to be employed than with nitrogen reduction to ammonia (open to air and water, and at room temperature and pressure). Once nitrite is produced, its central position in the nitrogen cycle allows its ready conversion to nitrogen species in other oxidation states using electrocatalysts that we are developing for these transformations (see figure below).

Electrosynthesis
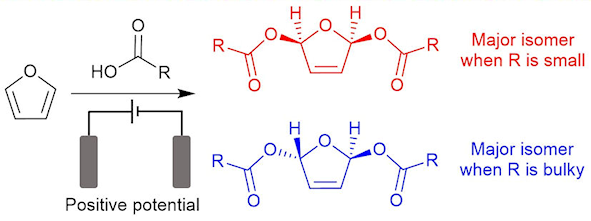
In addition to the electrosynthesis of ammonia and the nitrogen oxides, we are also interested in the application of electrochemistry to the synthesis of more complex organic compounds. We are currently developing both batch and flow-cells to allow us to explore a range of transformations of interest. See, for example, our 2019 paper in Royal Society Open Science.

Electrochemistry for Space Applications
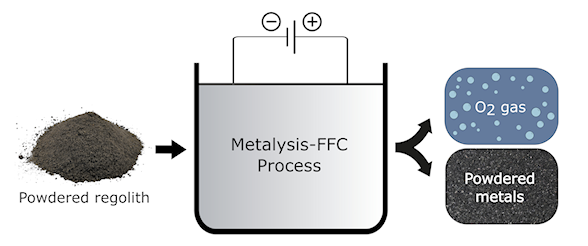
Electrochemistry could have significant benefits for the production of chemicals and materials where infrastructure is lacking. One such environment is space, where electrochemical methods could form a cornerstone of in situ resource utilization (ISRU), enabling human exploration of the cosmos. In this regard, we have demonstrated an electrochemical process that can extract oxygen from moonrock, co-generating metal alloys that could be used for construction on the lunar surface.

The electrochemical and spectroscopic properties of coordination complexes, and chemistry in molecular cages
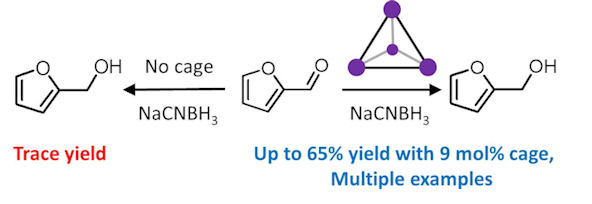
Finally, we also have fun making and examining the properties of new coordination complexes. Many of these complexes have fascinating electronic, redox, catalytic and spectroscopic properties, which we investigate with a battery of techniques (NMR, UV-vis, FTIR, Raman, powder and single crystal diffraction, electrochemistry, EPR, conductivity, mass spectrometry, computational techniques....) in order to work out how they do what they do! An area of special interest for us is trying to control the reactivity of substrates using, and/or within supramolecular coordination cages.