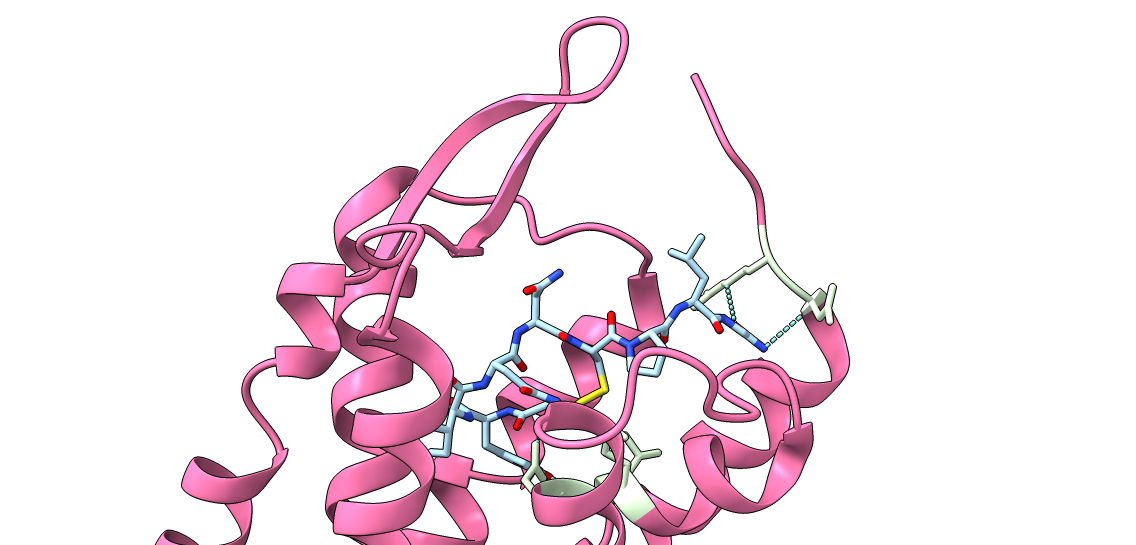
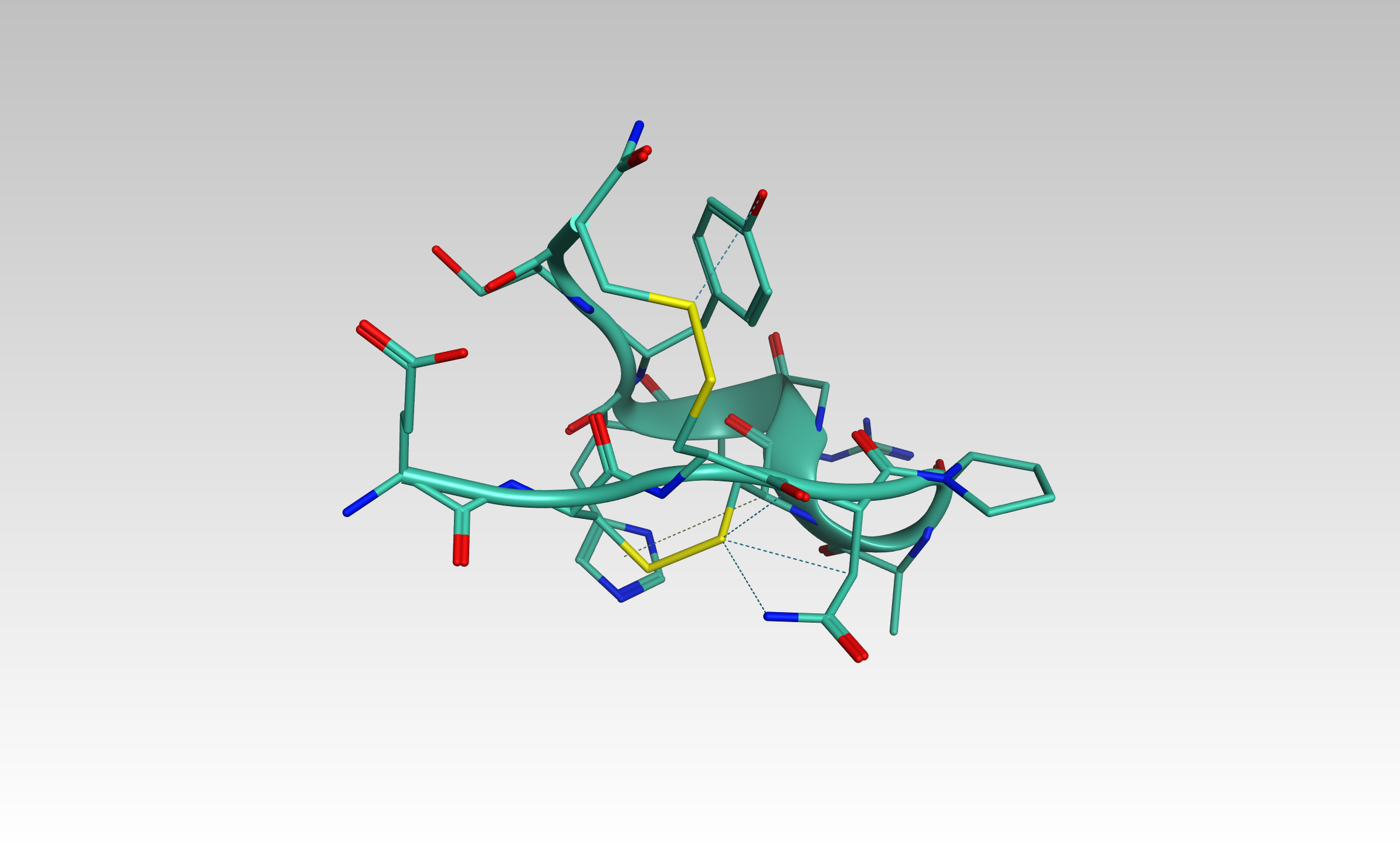
Our aim is to design and synthesise triazole stabilised analogues of macrocyclic peptides, in which the native disulfide bond is replaced by a triazole bridge.
We have worked on triazole analogues of a series of peptide targets with great success, such as Urotensin-II: a regulator of the urotensinergic system with a key role in cardiovascular health; conotoxin peptides: highly stable complex disulfide-bonded peptides; and Oxytocin: a peptide hormone which plays an essential role in labour and childbirth, along with love and social bonding.
Disulfide bonds feature in a great deal of peptides found in nature, with the disulfide bond providing improved stability to the peptide relative to its linear counterpart.
In disulfide-rich peptides the connectivity of disulfides can be essential in maintaining activity, providing challenges in their synthesis.
In order to achieve exquisite control over the folding an orthogonal protection strategy can be applied on solid support, with sequential deprotection and oxidation steps to fold the peptides into their bioactive form.
Work in the group focuses on the synthesis of disulfide-rich conotoxin peptides to develop a greater understanding of the nicotinic acetylcholine receptor.