2026
Expanding the scope of sustainable peptide synthesis through post-linear synthesis reactions
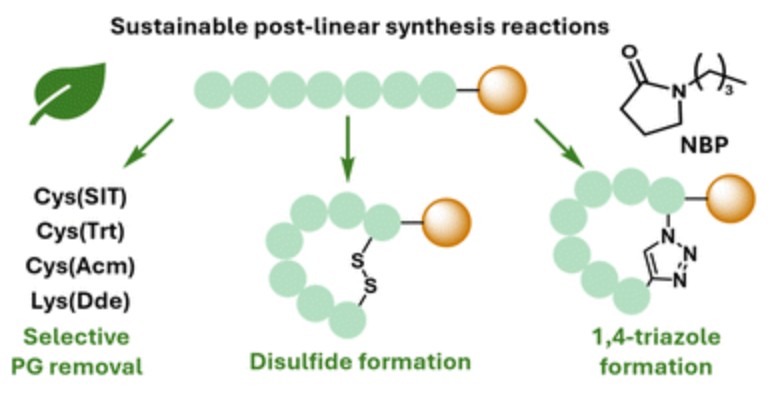
Michael A. Malone, Matilde De Francisco Craparotta, Kirsty I. M. Arnott, Andrew G. Jamieson and Nicola Wade.
Org. Biomol. Chem., 2026, Advance Article
DOI: 10.1039/D5OB01890K
Shaping Antimalarials: A Geometry-First Approach to PfCLK3 Covalent Inhibitors
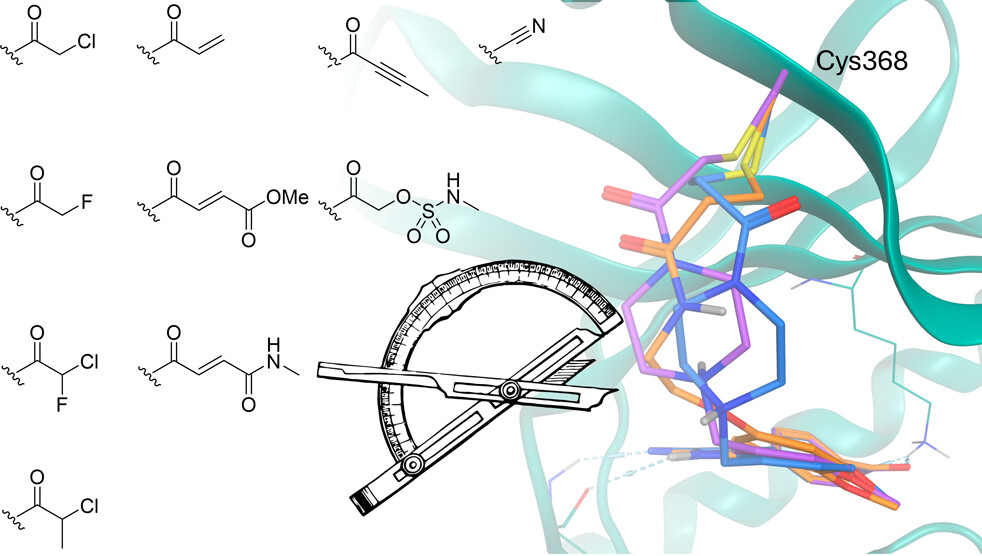
Skye B. Brettell, Carla Fuentes-Guerra Bustos, Saumya Sharma, Gillian Cann, Lauren V. Carruthers, Abbey Begen, Graeme Milligan, David J. Clarke, Andrew B. Tobin and Andrew G. Jamieson.
J. Med. Chem. 2026.
DOI: 10.1021/acs.jmedchem.5c03342
2025
On-Resin Synthesis and Late-Stage Functionalization of Macrocyclic Atosiban Mimetics via 5-Iodo-1,4-triazoles
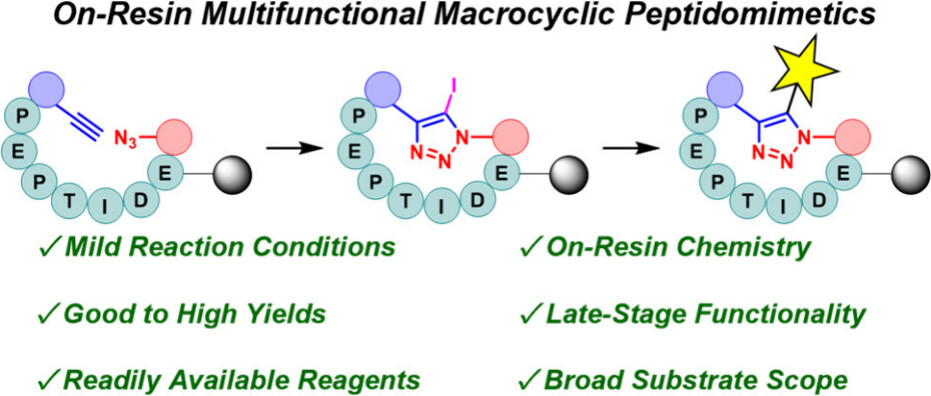
Oscar A. Shepperson, Michael A. Malone, Kirsty I. M. Arnott, Ryan J. Brown and Andrew G. Jamieson.
Org. Lett. 2025, 27, 40, 11249–11253.
DOI: 10.1039/D5MD00335K
Lysine targeting covalent inhibitors of malarial kinase PfCLK3
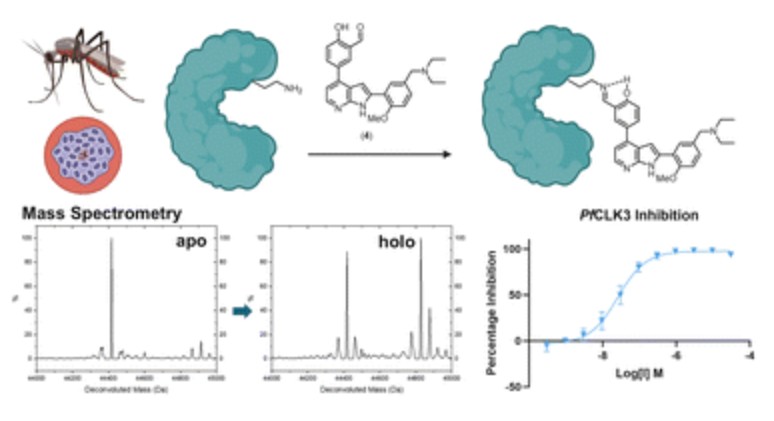
Skye B. Brettell, Gillian Cann, Abbey Begen, Saumya Sharma, Amit Mahindra, Lauren V. Carruthers, Graeme Milligan, David J. Clarke, Andrew B. Tobin and Andrew G. Jamieson.
RSC Med. Chem., 2025, 16, 3530-3540.
DOI: 10.1039/D4CB00288A
Raman active diyne-girder conformationally constrained p53 stapled peptides bind to MDM2 for visualisation without fluorophores
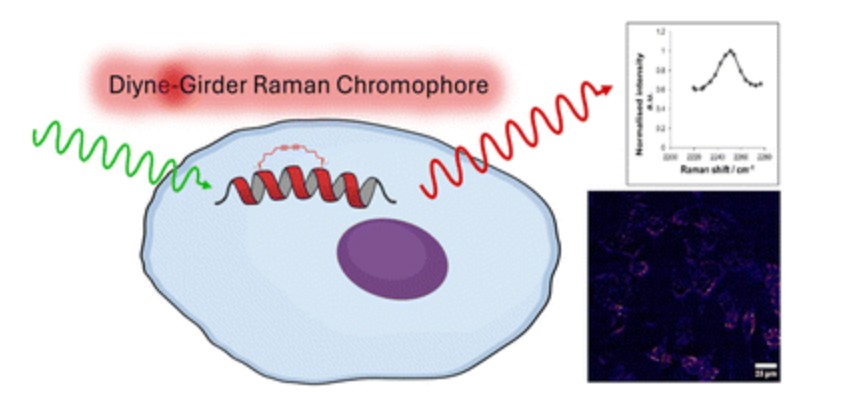
Danielle C. Morgan, Laura McDougall, Astrid Knuhtsen, Lori Buetow, Craig F. Steven, Oscar A. Shepperson, Danny T. Huang, Alison N. Hulme and Andrew G. Jamieson.
RSC Chem. Biol., 2025,6, 394-403.
DOI: 10.1039/D4CB00288A
2024
Targeting PfCLK3 with Covalent Inhibitors: A Novel Strategy for Malaria Treatment
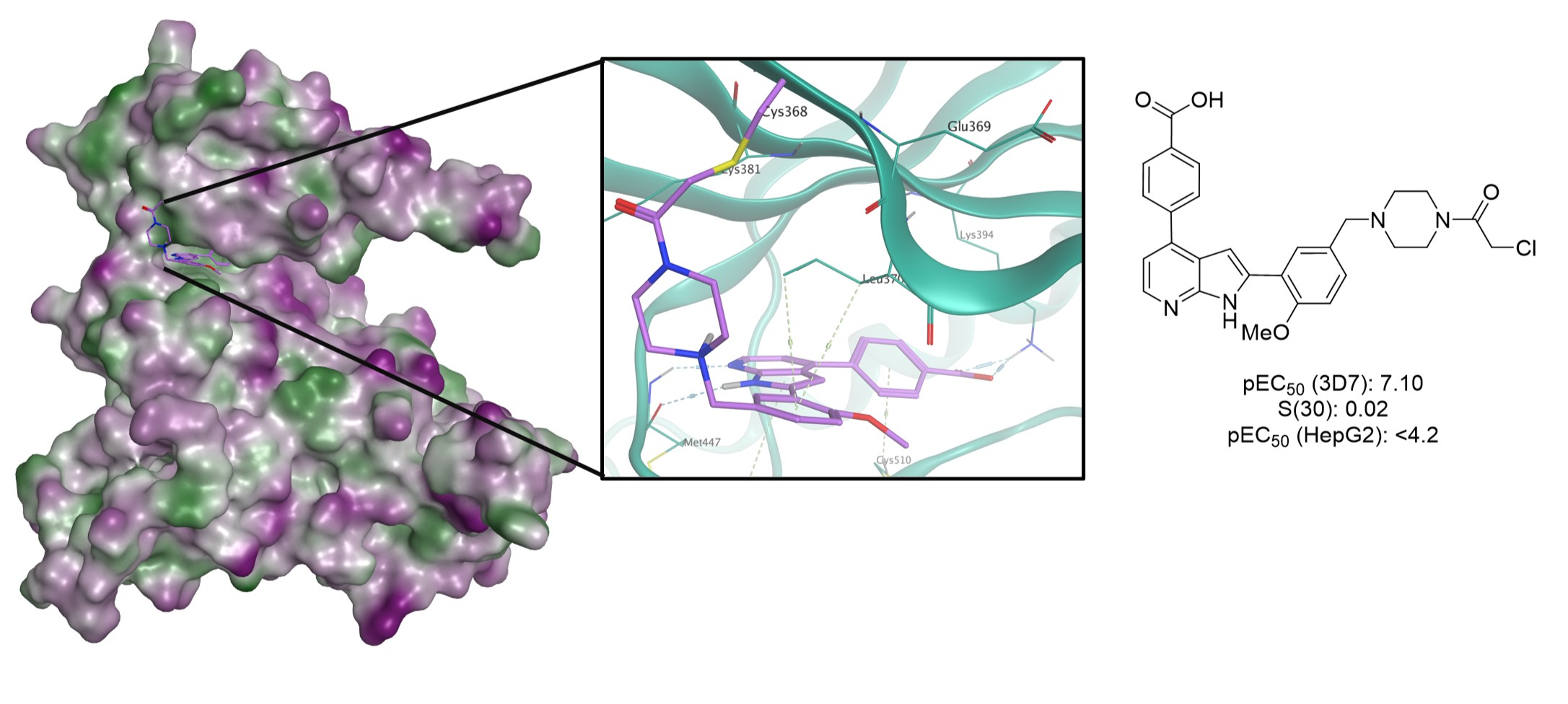
Skye B. Brettell, Omar Janha, Abbey Begen, Gillian Cann, Saumya Sharma, Niniola Olaniyan, Tamas Yelland, Alison J. Hole, Benazir Alam, Emily Mayville, Ross Gillespie, Michael Capper, David A. Fidock, Graeme Milligan, David J. Clarke, Andrew B. Tobin and Andrew G. Jamieson.
J. Med. Chem. 2024, 67, 21, 18895–18910.
DOI: 10.1021/acs.jmedchem.4c01300
2023
Rotamer-controlled dual emissive a-amino acids
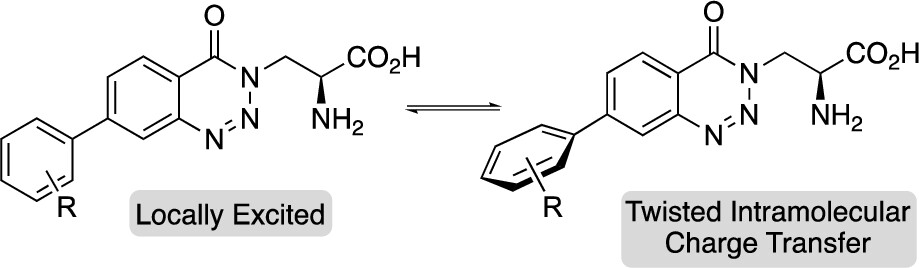
Rochelle McGrory, Danielle C. Morgan, Andrew G. Jamieson and Andrew Sutherland.
Org. Lett. 2023, 25, 31, 5844-5849.
DOI: 10.1021/acs.orglett.3c02112
Development of bifunctional, Raman active diyne-girder stapled a-helical peptides
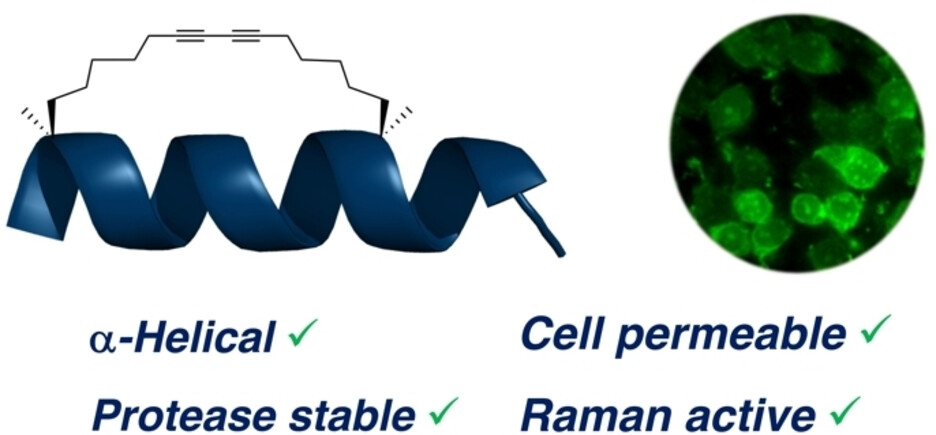
Danielle C. Morgan, Laura McDougall, Astrid Knuhtsen, and Andrew G. Jamieson.
Chem. Eur. J. 2023, 29, 41, e202300855.
DOI: 10.1002/chem.202300855
2022
Hydroxamic acid-modified peptide library provides insights into the molecular basis for the substrate selectivity of HDAC corepressor complexes.
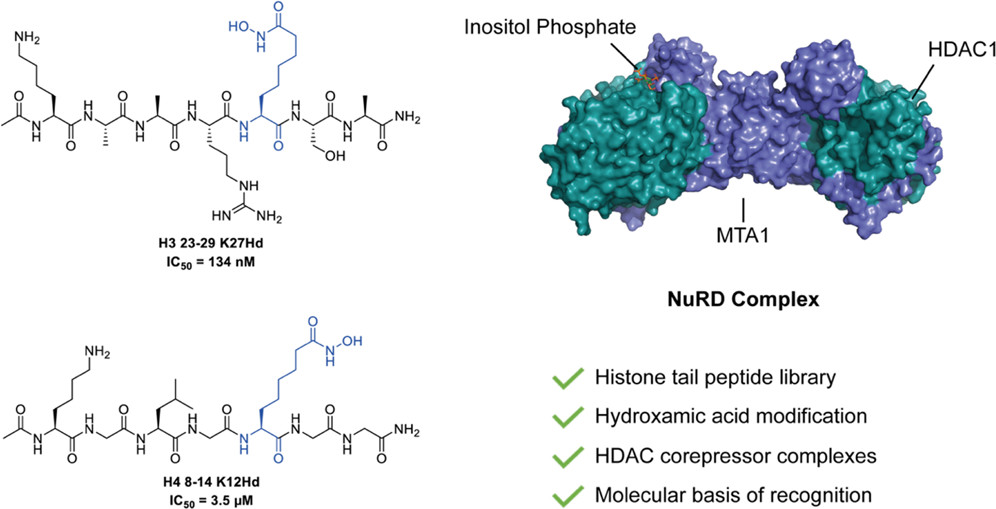
Lewis J. Archibald, Edward A. Brown, Christopher J. Millard, Peter J. Watson, Naomi S. Robertson, Siyu Wang, John W. R. Schwabe, and Andrew G. Jamieson.
ACS Chem. Biol., 2022, 17, 9, 2572-2582.
DOI: 10.1021/acschembio.2c00510
Investigating the Structure–Activity Relationship of 1,2,4-Triazine G-Protein-Coupled Receptor 84 (GPR84) Antagonists
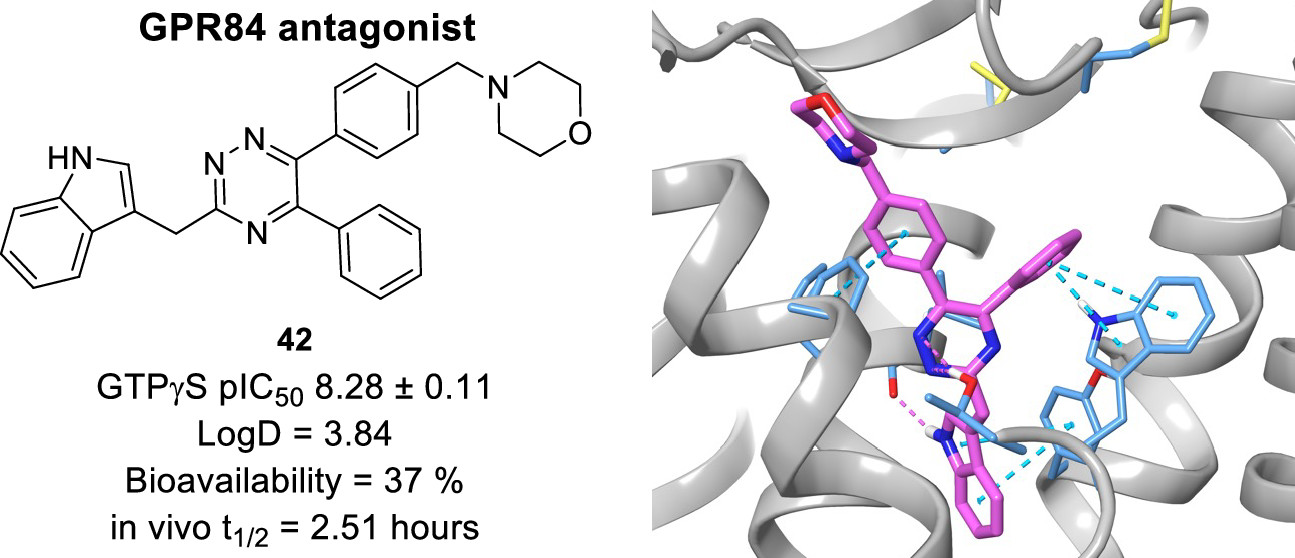
Amit Mahindra, Laura Jenkins, Sara Marsango, Mark Huggett, Margaret Huggett, Lindsay Robinson, Jonathan Gillespie, Muralikrishnan Rajamanickam, Angus Morrison, Stuart McElroy, Irina G. Tikhonova, Graeme Milligan, and Andrew G. Jamieson.
J. Med. Chem., 2022, 65, 16, 11270-11290.
DOI: 10.1021/acs.jmedchem.2c00804
Stabilization of the RAS:PDE6D Complex Is a Novel Strategy to Inhibit RAS Signaling
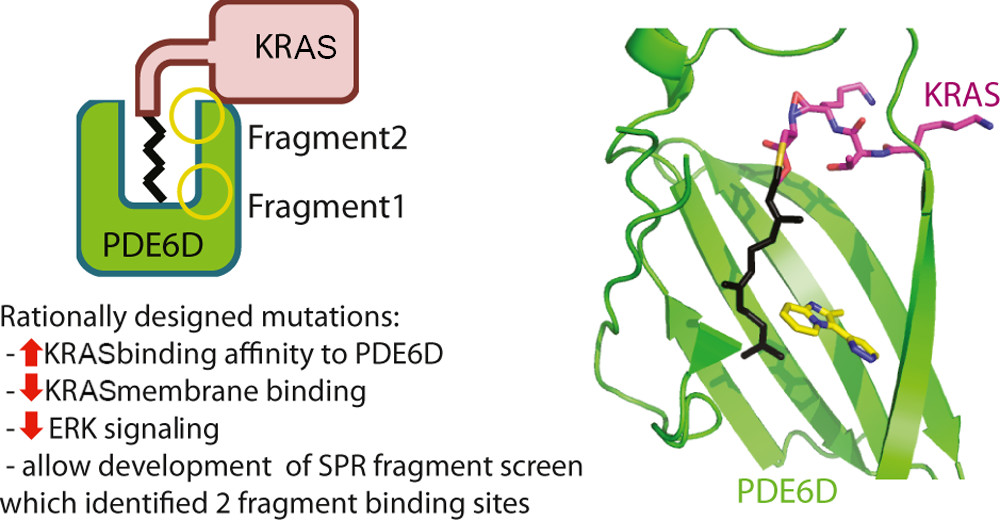
Tamas Yelland, Esther Garcia, Charles Parry, Dominika Kowalczyk, Marta Wojnowska, Andrea Gohlke, Matja Zalar, Kenneth Cameron, Gillian Goodwin, Qing Yu, Peng-Cheng Zhu, Yasmin ElMaghloob, Angelo Pugliese, Lewis Archibald, Andrew Jamieson, Yong Xiang Chen, Duncan McArthur, Justin Bower, and Shehab Ismail.
J. Med. Chem., 2022, 65, 3, 1898–1914.
DOI: 10.1021/acs.jmedchem.1c01265
2021
Peptides derived from the SARS-CoV-2 receptor binding motif bind to ACE2 but do not block ACE2-mediated host cell entry or pro-inflammatory cytokine induction
Amit Mahindra, Gonzalo Tejeda, Mario Rossi, Omar Janha, Imogen Herbert, Caroline Morris, Danielle C. Morgan, Wendy Beattie, Augusto C. Montezano, Brian Hudson, Andrew B. Tobin, David Bhella, Rhian M. Touyz, Andrew G. Jamieson, George S. Baillie, and Connor M. Blair.
PLoS ONE 2021, 16, 11, e0260283.
DOI: 10.1371/journal.pone.0260283
Stapled ACE2 peptidomimetics designed to target the SARS-CoV-2 spike protein do not prevent virus internalization
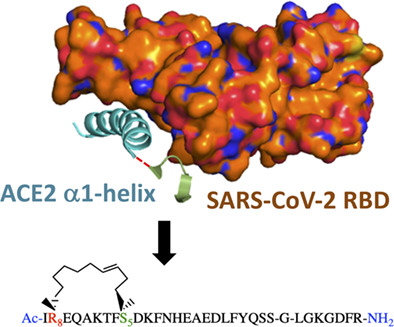
Danielle C. Morgan, Caroline Morris, Amit Mahindra, Connor M. Blair, Gonzalo Tejeda, Imogen Herbert, Matthew L. Turnbull, Gauthier Lieber, Brian J. Willett, Nicola Logan, Brian Smith, Andrew B. Tobin, David Bhella, George Baillie, Andrew G. Jamieson.
Pept. Sci., 2021, 113, 4, e24217.
DOI: 10.1002/pep2.24217
μ-Conotoxin KIIIA peptidomimetics that block human voltage-gated sodium channels
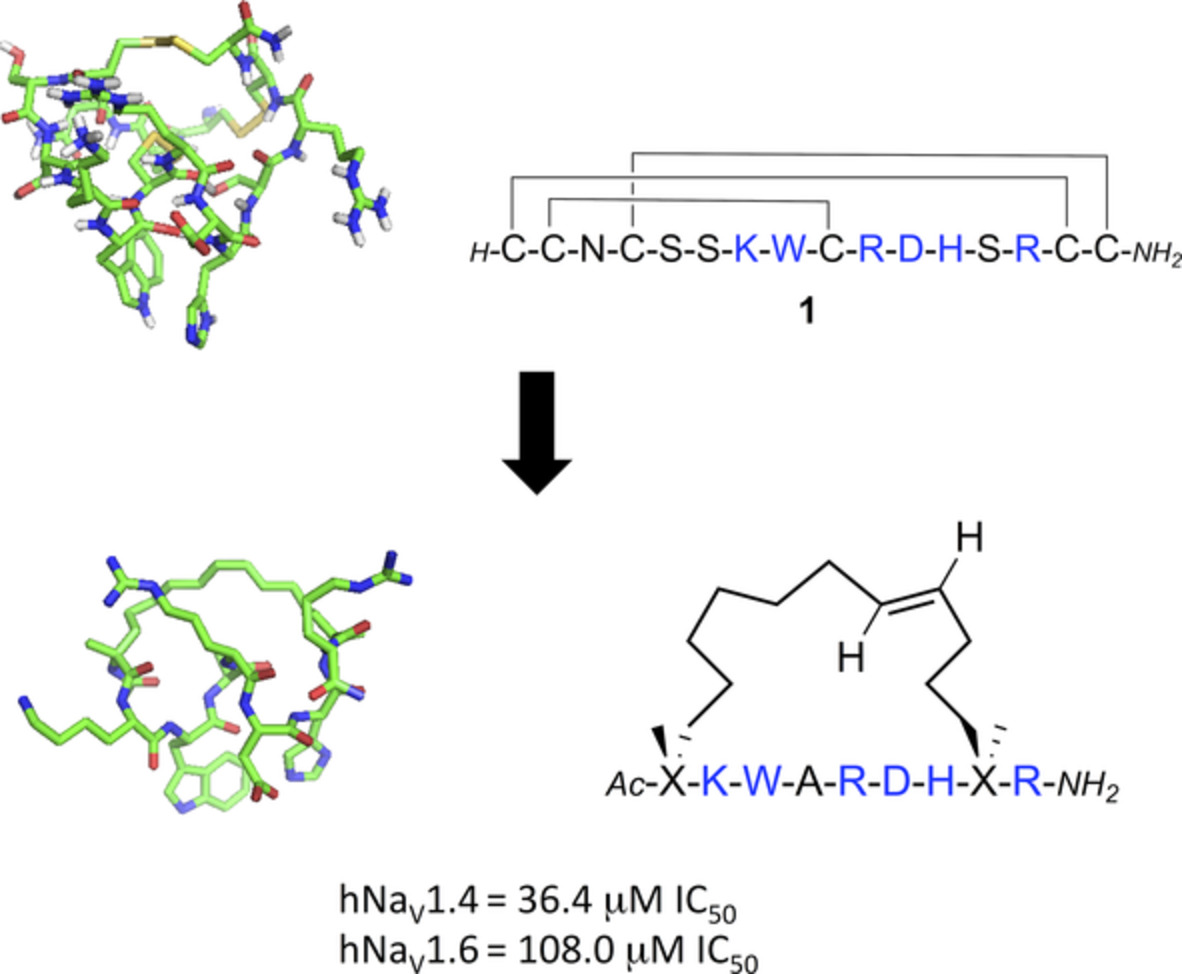
Astrid Knuhtsen, Rachel Whiting, Fergus S. McWhinnie, Charlotte Whitmore, Brian O. Smith, A. Christopher Green, Christopher M. Timperley, Kenneth I. Kinnear, Andrew G. Jamieson.
Pept. Sci., 2021, 113, 1, e24203.
DOI: 10.1002/pep2.24203
2020
Development of potent PfCLK3 inhibitors based on TCMDC-135051 as a new class of antimalarials
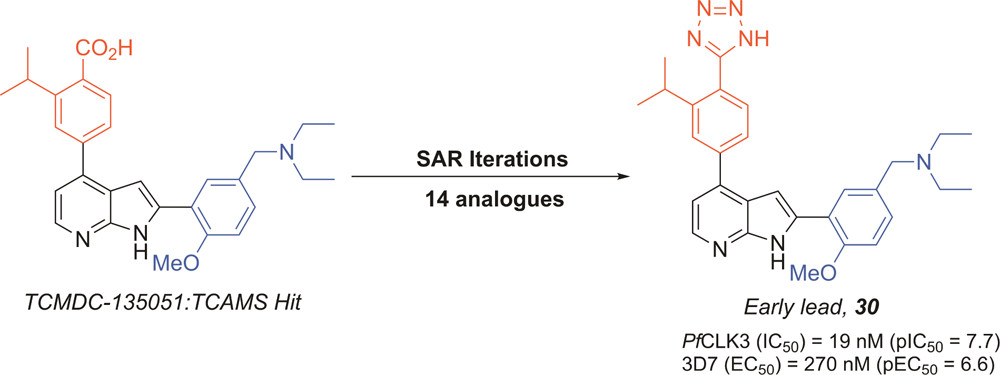
Amit Mahindra, Omar Janha, Kopano Mapesa, Ana Sanchez Azqueta, Mahmood M Alam, Alfred Amambua-Ngwa, Davies C Nwakanma, Andrew B Tobin, and Andrew G. Jamieson.
J. Med. Chem. 2020. 63, 17, 9300-9315.
DOI: 10.1021/acs.jmedchem.0c00451
2019
Validation of the protein kinase PfCLK3 as a multistage cross-species malarial drug target
Mahmood M. Alam, Ana Sanchez-Azqueta, Omar Janha, Erika L. Flannery, Amit Mahindra, Kopano Mapesa,
Aditya B. Char, Dev Sriranganadane, Nicolas M. B. Brancucci, Yevgeniya Antonova-Koch, Kathryn Crouch,
Nelson Victor Simwela, Scott B. Millar, Jude Akinwale, Deborah Mitcheson, Lev Solyakov, Kate Dudek,
Carolyn Jones, Cleofé Zapatero, Christian Doerig, Davis C. Nwakanma, Maria Jesús Vázquez,
Gonzalo Colmenarejo, Maria Jose Lafuente-Monasterio, Maria Luisa Leon, Paulo H. C. Godoi, Jon M. Elkins,
Andrew P. Waters, Andrew G. Jamieson, Elena Fernández Álvaro, Lisa C. Ranford-Cartwright, Matthias Marti,
Elizabeth A. Winzeler, Francisco Javier Gamo and Andrew B. Tobin.
Science, 2019, 365, 6456, eaau1682.
DOI: 10.1126/science.aau1682
Synthesis of HDAC Substrate Peptidomimetic Inhibitors Using Fmoc Amino Acids Incorporating Zinc-Binding Groups
Amit Mahindra, Christopher J. Millard, Iona Black, Lewis J. Archibald, John W. R. Schwabe and Andrew G. Jamieson.
Organic Letters, 2019, 21, 9, 3178-3182.
DOI: 10.1021/acs.orglett.9b00885
Synthesis and fluorescent properties of beta-pyridyl alpha-amino acids.
Harkiss, A. H., Bell, J. D., Knuhtsen, A., Jamieson, A. G. and Sutherland, A.
Journal of Organic Chemistry, 2019, 84, 5, 2879-2890.
DOI: 10.1021/acs.joc.9b00036
Friends or foes? Emerging impacts of biological toxins.
Clark, G. C., Casewell, N. R., Elliott, C. T., Harvey, A. L., Jamieson, A. G. , Strong, P. N. and Turner, A. D.
Trends in Biochemical Sciences, 2019, 44, 4, 365-379.
DOI: 10.1016/j.tibs.2018.12.004
2018
alpha-conotoxin GI triazole-peptidomimetics: potent and stable blockers of a human acetylcholine receptor
Astrid Knuhtsen, Charlotte Whitmore, Fergus S. McWhinnie, Laura McDougall, Rachel Whiting, Brian O. Smith, Christopher M. Timperley, A. Christopher Green, Kenneth I. Kinnear and Andrew G. Jamieson*.
Chemical Science, 2019, 10, 1671-1676.
DOI: 10.1039/C8SC04198A
Histone deacetylase (HDAC) 1 and 2 complexes regulate both histone acetylation and crotonylation in vivo.
Kelly, R.D.W., Chandru, A., Watson, P.J., Song, Y., Blades, M., Robertson, N.S., Jamieson, A.G. , Schwabe, J.W.R. and Cowley, S.M.
Scientific Reports, 2018, 8(1), 14690.
DOI: 10.1038/s41598-018-32927-9
Lipopeptidomimetics derived from teixobactin have potent antibacterial activity against Staphylococcus aureus
Georgina C. Girt, Amit Mahindra, Zaaima J. H. Al Jabri, Megan De Ste Croix, Marco R. Oggionic and Andrew G. Jamieson*.
Chem. Commun., 2018, 54, 2767-2770.
DOI: 10.1039/C7CC06093A
Enzymatically-stable oxetane-based dipeptide hydrogels.
Laura McDougall, Emily R. Draper, Jonathan D. Beadle, Michael Shipman, Piotr Raubo, Andrew G. Jamieson* and Dave J. Adams*.
Chem. Commun., 2018, 54, 1793-1796.
DOI: 10.1039/C7CC09701H
2017
Solid-phase synthesis of oxetane modified peptides.
Jonathan D. Beadle, Astrid Knuhtsen, Alex Hoose, Piotr Raubo, Andrew G. Jamieson*, and Michael Shipman*.
Org. Lett., 2017, 19, 12, 3303–3306.
DOI: 10.1021/acs.orglett.7b01466.
Urotensin-II peptidomimetic incorporating a non-reducible 1,5-triazole disulfide bond reveals a pseudo-irreversible covalent binding mechanism to the urotensin G-protein coupled receptor.
Pacifico S, Kerckhoffs A, Fallow AJ, Foreman RE, Guerrini R, McDonald J, Lambert DG, Jamieson AG.
Org Biomol Chem. 2017, 15, 21, 4704-4710.
DOI: 10.1039/c7ob00959c.
2016
Insights into the activation mechanism of class I HDAC complexes by inositol phosphates
Peter J. Watson, Christopher J. Millard, Andrew M. Riley, Naomi S. Robertson, Lyndsey C. Wright, Himali Y. Godage, Shaun M. Cowley, Andrew G. Jamieson, Barry V. L. Potter & John W. R. Schwabe.
Nat. Comm., 2016, 7, 11262.
DOI: 10.1038/ncomms11262.
A TPX2 proteomimetic has enhanced affinity for Aurora-A due to hydrocarbon stapling of a helix.
Yana K. Rennie†, Patrick J. McIntyre, Tito Akindele, Richard Bayliss*, and Andrew G. Jamieson*.
ACS Chem. Biol., 2016, 11, 12, pp 3383–3390.
DOI: 10.1021/acschembio.6b00727.
A heme-binding domain controls regulation of ATP-dependent potassium channels
Mark J. Burton, Sofia M. Kapetanaki, Tatyana Chernova, Andrew G. Jamieson, Pierre Dorlet, Jérôme Santolini, Peter C. E. Moody, John S. Mitcheson, Noel W. Davies, Ralf Schmid, Emma L. Raven and Nina M. Storey.
PNAS 2016, 113, 14, 3785-3790.
DOI: 10.1073/pnas.1600211113.
2015
Regulation of protein–protein interactions using stapled peptides.
Robertson N, Jamieson A.
Reports in Organic Chemistry, 2015, 5, 65-74.
DOI: 10.2147/ROC.S68161.
Insights into the recruitment of class IIa Histone Deacetylases (HDACs) to the SMRT/NCoR transcriptional repression complex.
Hudson GM, Watson PJ, Fairall L, Jamieson AG, Schwabe JW.
J Biol. Chem., 2015, 290, 29, 18237-44.
DOI: 10.1074/jbc.M115.661058.
2014
Robust asymmetric synthesis of unnatural alkenyl amino acids for conformationally constrained alpha-helix peptides.
Boris Aillard, Naomi S. Robertson, Adam R. Baldwin, Siobhan Robins and Andrew G. Jamieson.
Org. Biomol. Chem., 2014, 12, 8775-8782.
DOI: 10.1039/C4OB01832J.
The ansamycin antibiotic, Rifamycin SV, inhibits BCL6 transcriptional repression and forms a complex with the BCL6-BTB/POZ domain.
Sian E. Evans, Benjamin T. Goult, Louise Fairall, Andrew G. Jamieson, Paul Ko Ferrigno, Robert Ford, John W. R. Schwabe, Simon D. Wagner
PLoS ONE, 9, 3, e90889.
DOI: 10.1371/journal.pone.0090889.
2013
Peptide scanning for studying structure-activity relationships in drug discovery.
Jamieson AG, Boutard N, Sabatino D, Lubell WD.
Chem. Biol. Drug Des., 2013, 81, 1, 148-65.
DOI: 10.1111/cbdd.12042..
2012
A novel dihydro-pyrazolo(3,4d)(1,2,4)triazolo(1,5a)pyrimidin-4-one (AJ23) is an antagonist at adenosine A1 receptors and enhances consolidation of step-down avoidance.
Harvey, A. L., Young, L. C., Kornisiuk, E., Snitcofsky, M., Colettis, N., Blanco, C., Jerusalinsky, D., Jamieson, A. G. , Hartley, R. C. and Stone, T. W.
Behavioural Brain Research, 234, 2, 184-191.
DOI: 10.1016/j.bbr.2012.06.023.
A 1,3-phenyl-linked hydantoin oligomer scaffold as a β-strand mimetic.
Andrew G. Jamieson,* David Russell and Andrew D. Hamilton*.
Chem. Commun., 2012, 48, 3709-3711.
DOI: 10.1039/C2CC30295K.
2010
Insertion of multiple α-amino γ-lactam (Agl) residues into a peptide sequence by solid-phase synthesis on synphase lanterns.
Luisa Ronga, Andrew G. Jamieson, Kim Beauregard, Christiane Quiniou, Sylvain Chemtob, William D. Lubell.
Biopolymers, 2010, 94, 2, 183-191.
DOI: 10.1002/bip.21288.
Structure-activity analysis of the growth hormone Secretagogue GHRP-6 by α- and β-amino γ-lactam positional scanning.
Nicolas Boutard, Andrew G. Jamieson, Huy Ong, William D. Lubell.
Chemical Biology and Drug Design, 75, 1, 40-50.
DOI: 10.1111/j.1747-0285.2009.00913.x.
α-Amino-β-hydroxy-γ-lactam for constraining peptide Ser and Thr residue conformation.
Daniel J. St-Cyr, Andrew G. Jamieson and William D. Lubell*.
Org. Lett., 2010, 12, 8, 1652–1655.
DOI: 10.1021/ol1000582.
2009
Positional scanning for peptide secondary structure by systematic solid-phase synthesis of amino lactam peptides.
Andrew G. Jamieson, Nicolas Boutard, Kim Beauregard, Mandar S. Bodas, Huy Ong, Christiane Quiniou, Sylvain Chemtob and William D. Lubell.
J. Am. Chem. Soc., 2009, 131, 22, 7917–7927.
DOI: 10.1021/ja9010628.
2007
Ether-directed palladium(II)-catalysed aza-Claisen rearrangements: studies on the origin of the directing effect.
Jamieson, A. G. and Sutherland, A.
Tetrahedron, 2007, 63, 2123-2131.
DOI: 10.1016/j.tet.2006.12.067
Ether-directed, stereoselective aza-Claisen rearrangements: Synthesis of the piperidine alkaloid, alpha-conhydrine.
Jamieson, A. G. and Sutherland, A.
Organic Letters, 2007, 9, 1609-1611.
DOI: 10.1021/ol070424z
2006
Palladium(II)-catalysed rearrangement reactions.
Fanning, K. N., Jamieson, A. G. and Sutherland, A.
Current Organic Chemistry, 10, 9, 1007-1020.
DOI: 10.2174/138527206777435490
Scope and limitations of ether-directed, metal-catalysed aza-Claisen rearrangements; improved stereoselectivity using non-coordinating solvents.
Jamieson, A. G. and Sutherland, A.
Organic and Biomolecular Chemistry, 2006, 4, 2932-2937.
DOI: 10.1039/B607014K
2005
Stereoselective beta-hydroxy-alpha-amino acid synthesis via an ether-directed, palladium-catalysed aza-Claisen rearrangement.
Fanning, K. N., Jamieson, A. G. and Sutherland, A.
Organic and Biomolecular Chemistry, 3, 3749-3756.
DOI: 10.1039/B510808J
A highly stereoselective ether directed palladium catalysed aza-Claisen rearrangement.
Jamieson, A. G. and Sutherland, A.
Organic and Biomolecular Chemistry, 3, 735-736.
DOI: 10.1039/B501346C
2004
The first enantioselective synthesis of the amino acid, (2S,3S,4R)-gamma-hydroxyisoleucine using a palladium(II) catalysed 3,3-sigmatropic rearrangement.
Jamieson, A. G. , Sutherland, A. and Willis, C. L.
Organic and Biomolecular Chemistry, 2, 808-809.
DOI: 10.1039/B401076K